
The cover image was created by the transcriber and is placed in the public domain.
The Project Gutenberg EBook of Roentgen Rays and Phenomena of the Anode and Cathode., by Edward P. Thompson This eBook is for the use of anyone anywhere in the United States and most other parts of the world at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this eBook or online at www.gutenberg.org. If you are not located in the United States, you'll have to check the laws of the country where you are located before using this ebook. Title: Roentgen Rays and Phenomena of the Anode and Cathode. Author: Edward P. Thompson Contributor: William A. Anthony Release Date: October 9, 2020 [EBook #63422] Language: English Character set encoding: UTF-8 *** START OF THIS PROJECT GUTENBERG EBOOK ROENTGEN RAYS AND PHENOMENA *** Produced by deaurider, Barry Abrahamsen, and the Online Distributed Proofreading Team at https://www.pgdp.net (This file was produced from images generously made available by The Internet Archive)

The cover image was created by the transcriber and is placed in the public domain.

In addition to the illustrated feature for exhibiting the nature and practical application of X-rays, and for simplifying the descriptions, the book involves the disclosure of the facts and principles relating to the phenomena occurring between and around charged electrodes, separated by different gaseous media at various pressures. The specific aim is the treatment of the radiant energy developed within and from a discharge tube, the only source of X-rays.
Having always admired the plan adopted by German investigators in publishing accounts of their experiments by means of numbered paragraphs containing cross-references and sketches, the author has likewise treated the investigations of a large number of physicists. The cross-references are indicated by the section sign (§). By reference, the analogy, contrast, or suggestiveness may be meditated upon. All knowledge of modern physics is based upon experiments as the original source. Inasmuch as many years may be expected to elapse before the innumerable peculiarities of the electrical discharge will be reduced to a pure science, and also in order that the contents of the book may be of value in the future as well as at present, the characteristic experiments of electricians and scientists are described, in general, by reference to their object, the apparatus used, the result, the inferences of the experimenter, and the observations of cotemporaneous or later physicists, together with a presentation here and there of theoretical matters and allusion to practical applications.
The classes of reader to which the book is adapted may best be known, of course, after perusal, but some advance intimation of the kind that the author had in view may be desired. Let it be known that, first, the student and those generally interested in science iiought to be able to comprehend the subject-matter, because experiments are described, which are always the simplest means (e.g., in a popular lecture) for explaining the wonders of any given scientific principles or facts. Thus did Crookes, Tyndall, Thomson (both Kelvin and J. J.), Hertz, etc., disseminate knowledge—by describing their researches and reasoning thereon.
In view of the tremendous amount of experimenting which has been carried on during the past few years in connection with the electric discharge, it was difficult to determine just how far back to begin (without starting at the very beginning), so that the student and general reader, whose object is to become acquainted especially with the properties of cathode and X-rays, might better understand them. The author realized that it was necessary to go back further and further in this department of science, and he could not easily stop until he had reached certain investigations of Faraday, Davy, Page, and others, which are briefly noticed in an introductory sense. Take, for example, the inaction of the magnet upon X-rays in open air. § 79. Of course, it would be of interest for the student to know about Lenard’s investigations relating to the action of the magnet upon cathode rays inside of the observing tube. § 72a. It would follow, further, that he would desire to know about Crookes’ experiment relating to the attraction of the magnet upon cathode rays within the tube. § 59. In order that he might not infer that Crookes was the first to investigate the action of the magnet upon the discharge, it was evident that the book could be made of greater value by relating the experiments of Prof. J. J. Thomson as to the discharge across and along the lines of magnetic force, § 31, and Plücker’s experiment on the action of the magnet upon the cathode column of light. § 30. The interest became increased, instead of diminished, by noting De la Rive’s experiment on the rotation of the luminous effect of the discharge by means of the magnet. § 29. Being now quite impossible to stop, Davy’s electric arc and magnetic action upon the same had to be alluded to, at least briefly. § 28. On the other hand, the very earliest experiments with the discharge in rarefied air are not described—occurring as remotely as the eighteenth century—so ably treated of in Park Benjamin’s work. Those facts that have some mutual iiibearing are brought forward to serve as stepping-stones to the investigation of cathode and X-rays.
Secondly, the author often imagined that he was writing in behalf of the surgeon and physician and those who intend to experiment, especially when he found in his investigations of recent publications descriptions in detail of the electrical apparatus employed in experimenting with X-rays. He improved the opportunity of repeating the statements of the difficulties, and how they were overcome; also, the precautions necessary to be taken, and, besides, the kind of discharge tubes and apparatus best adapted for particular kinds of experiments. The chapter on applications in diagnosis and anatomy, etc., is of especial interest to physicians.
Thirdly, as the discovery of the Roentgen rays has established a new department of photography, those who are interested in this art may be benefited by the results and suggestions disclosed in connection with photographic plates, time of exposure, adjuncts for best results, precautions for obtaining sharp shadows, and steps of the process, from beginning to end, for carrying on the operation.
Fourthly, expert physicists and electricians, professors, etc., need something that the above classes do not, and this is the reason why the author has not assumed the burden of carrying any line of thought or theory from the beginning to the end of the treatise, nor has he made the book in any way a personal matter by criticising experiments, nor even by favoring the views of one over the other, unless it is in an exceptional case here and there; but in each instance the investigator’s name is given, and that of the publication in which the account may be found, so that the scientist may refer thereto to test the correctness of the author’s version of the matter, or to learn the nature of the minute details and circumstances.
The author suggests that the study of the phenomena of the discharge tube would not be amiss in scientific schools and colleges. He argues that in view of all experimenters in this line having been made enthusiastic and fascinated by reason of (1) the beautiful effects, (2) the field being always open to new discoveries, (3) the direct practical and theoretical bearing of the peculiar actions upon other departments of electricity, light, heat, and magnetism, (4) the pleasure in attempting to obtain results reported by others, and especially ivthe large amount of valuable theoretical and practical instruction resulting therefrom, by repeating the experiments or studying them, and (5) the possible applications of the discharge tube in connection with electric lighting and in the new department of sciagraphy by X-rays, and for other good and valuable considerations—it follows that students who have been through or who are studying a text-book of physics and electricity would be greatly benefited by a course in the discharge-tube phenomena.
In view of the large amount of dictation necessary in order to complete the work in such a short period, and in order that the subject-matter might involve the treatment of the latest work of the French and German as well as of the English and American, and inasmuch as the journals of the latter did not always contain complete translations and, for better service in behalf of the readers, the authorship was shared with others, and, therefore, much credit is due to Prof. Anthony for final chapter, to Mr. Louis M. Pignolet for assistance in connection with French periodicals and academy papers (§ § 63a, 84, 99, 101a, 103a, 112a, 124a, 128, at end, 139a, 154, 155, 156, 157, 158, and 159); to Mr. N. D. C. Hodges, formerly editor and proprietor of Science, who obtained some pertinent accounts, (§ 97a, 97b, 99A, B, C, D, to 99T, inclusive) by investigations of recent literature at the Astor Library, New York; and also to Mr. Ludwig Gutmann (Member American Institute of Electrical Engineers) for a few translations from the German.
Credit is given in each instance to all societies and publications by naming them in the respective paragraphs herein. In nearly every case the author prepared his material from original articles and papers contributed by the investigators to the societies or periodicals.
The author has prepared himself to withstand, with about half as much patience as he expects will be required, all criticisms based upon disappointments which may be experienced by the true, or the alleged true, first discoverer of any particular property of the electric discharge not duly credited. He has been particular in presenting knowledge as to physical facts and principles, but not equally, perhaps, as to the originator of the experiment, or as to the actual first discoverer, for the simple reason that the book is in no sense a history vnot a biography. Where the paragraph has been headed, for example, “Swinton’s Experiment,” it means that that party (according to the article purporting to be written by him) made that experiment. Some one else may have made exactly the same experiment previously, yet the instruction is equally as valuable as though the researches of the first discoverer had been related. On the other hand, the author has never had any intention of giving credit to the wrong party. The dates in the captions indicate the general chronological order in behalf of those thus interested. With this explanation, it is thought that the claimants will be much more lenient in their criticisms concerning priority of discovery. While the developments have generally followed each other historically, as well as appropriately for the purpose of instruction, yet now and then it was preferable to place the description of a comparatively recent experiment in conjunction with some description of an experiment made at a much earlier date. For this reason, also, the book is not of a chronological nature. The subject-matter, as usual, is divided into chapters, but the sections are to be considered as subordinate chapters, having different shades of meaning, and the one not necessarily bearing a direct relation to the contents of its neighbor, but as, in a novel or a treatise on geometry, having its important part to play in conjunction with some later or preceding section.
| § 1. | Secondary Current by Induction. No Increased E. M. F. | Faraday |
| 2. | Electric Spark and Increased E. M. F. by Induced Current. | Page |
| 3. | Spark in Secondary Increased by Condenser in Primary. | Fizeau |
| 4. | Atmosphere around an Incandescent Live Wire. | Vincintini |
| 5. | Magnetizing Radiations from an Electric Spark. | Henry |
| 6. | Arcing Metals at Low Voltage. | Faraday |
| 7. | Non-arcing Metals at High Voltage. Practical Application. | Wurts |
| 8. | Duration of Spark Measured. | Wheatstone |
| 8a. | Discharge—Intermittent, Constant, and Oscillatory—by Variation of Resistance. | Feddersen |
| 9. | Musical Note by Discharge with Small Ball Electrodes. Invisible Discharge. | Faraday |
| 9a. | Pitch of Sound Changed by Approach of Conductor Connected to Earth. | Faraday and Mayer |
| 10. | Brush Discharge. Color. Striæ. Nitrogen Best Transmitter of a Spark, and its Practical Bearing in Atmospheric Lightning. Cathode Brushes in Different Gases. | Faraday |
| 11. | Glow by Discharge. Glow Changed to Spark. Motion of Air. Apparent Continuous Discharge during Glow. | Faraday |
| 12. | Spark. Solids Perforated. | Lullin |
| 13. | Spark. Glass Perforated. Holes Close Together. Practical Application for Porous Glass. | Fage |
| 14 and 14a. | Spark. Penetrating Power. Conducting Power of Gas. Relation of E. M. F. to Pressure of Gases. Discharge through Hydrogen Vacuum Continued with Less Current than that Required to Start it. | Knochenhaurer, Boltzmann, Thomson (Kelvin), Maxwell, Varley, Harris, and Masson |
| 15. | Dust Particles or Rust on the Electrodes Hasten Discharge. | Gordon |
| 16. | Where the Distance is Greater, the Dielectric Strength is Smaller, Both Distances Being Minute. | Thomson (Kelvin) |
| 17. | Discharge through Gases under Very High Pressures. Increased Dielectric Strength. | Cailletet |
| 18. | Discharges in Different Chemical Gases Variably Resisted. | Faraday |
| 19. | Gas as a Conductor. Molecule for Molecule, its Conductivity Greater than that for Gases. | Thomson, J. J. |
| vii20. | Relation of Light to Electricity. The Square Root of the Dielectric Capacity Equal to the Refractive Index. | Boltzmann, Gibson, Barclay, Hopkinson, and Gladstone |
| 21. | Hermetically Sealed Discharge Tubes with Platinum Leading-in Wires. | Plücker and Geissler |
| 22. | Luminosity of Discharge Tubes Produced by Rubbing. Increased by Low Temperature. | Geissler |
| 23. | Different Vacua Needed for Luminosity by Friction and by Discharge. | Alvergniat |
| 24. | Phenomena of Discharge around the Edges of an Insulating Sheet. | Steinmetz |
| 25. | Highest Possible Vacuum Considered as a Non-conductor. | Morgan |
| 26. | Constant Potential at the Terminals of a Discharge Tube. | De La Rue and Müller |
| 26a. | Polarity of Discharge-tube Terminals in Secondary of Ruhmkorff Coil. Mathematical Deductions. | Klingenberg |
| 27. | Pressure in Discharge Tube Produced by a Spark. | Kinnersley, Harris, and Riess |
| 28. | Actions of Magnetism upon the Arc and Flame. | Davy, Bancalari, and Quet |
| 29. | Rotation of Luminous Discharge by a Magnet. Application in Explaining Aurora Borealis. | De La Rive |
| 30. | Action of Magnet on the Cathode Light. Relations Different according to the Position Relatively to the Magnetic Lines of Force. | Plücker and Hittorf |
| 31. | Discharge Retarded Across, and Accelerated Along, the Lines of Magnetic Force. | Thomson, J. J. |
| 32. | Resistance of Luminosity of the Discharge Afforded by a Thin Diaphragm. | Thomson, J. J. |
| 33. | Forcing Effect of the Striæ at a Perforated Diaphragm. | Solomons |
| 34. | Electric Images. | Riess |
| 35. | Electrographs on Photographic Plate by Discharge. | Sanford and McKay |
| 36. | Positive and Negative Dust Pictures upon Lines Drawn by Electrodes. | Lichtenberg |
| 36a. | Photo-electric Dust Figures. | Hammer |
| 36b. | Dust Portrait. | Hammer |
| 37. | Electrical Images by Discharge Developed by Condensed Moisture. | Karsten |
| 37a. | Magnetographs. | McKay |
| 38. | Bas-relief Facsimiles by Electric Discharge. | Piltchikoff |
| 39. | Distillation of Liquids by Discharge. | Gernez |
| 40. | Striæ. Black Prints on Walls of Tube. | De La Rue and Müller |
| 41. | Discharge Tube in Primary Current. Striæ. Least E. M. F. Required. | Gassiot |
| 42. | Current Interrupted Inside of Discharge Tube instead of Outside. | Poggendorff |
| 43. | Source of Striæ at the Anode. Color Changed by Change of Current. | De La Rue and Müller |
| 44. | Dark Bands by Small Discharges Disappear on Increase of Current, and Appear Again by Further Increase. | Solomons |
| 45. | Motion of Striæ. Method of Obtaining Motion when Desired and of Stopping the Same. | Spottiswoode |
| 46. | Motion of Striæ Checked at the Cathode. Tube, 50 ft. Long. The Anode the Starting-point. | Thomson, J. J. |
| 47. | Electrolysis in Discharge Tube. | Thomson, J. J. |
| 48. | Heat Striæ without Luminous Striæ. | De La Rue and Müller |
| 49. | Sensitive State. Method of Obtaining. Telephone Used to Prove Intermissions. | Spottiswoode and Moulton |
| 49a. | Cause of Sensitive State Detected by Telephone. | Spottiswoode and Moulton |
| 50. | Sensitive State Illustrated by a Flexible Conductor within the Discharge Tube. | Reitlinger and Urbanitzky |
| 51. | System of Operating Discharge Tubes. Excessively High Potential and Enormous Frequency. | Tesla |
| 52. | Discharge-tube Phenomena by Self-induced Currents. | Moore |
| 53. | Dark Space around the Cathode. | Crookes |
| 54. | Relation of Vacuum to Phosphorescence. | Crookes |
| 55. | Phosphorescence of Objects within Discharge Tube. | Crookes |
| 56. | Darkness and Luminosity in the Arms of a V Tube. | Crookes |
| 57. | Cathode Rays Rectilinear within the Discharge Tube. | Crookes |
| 58. | Shadow Cast within the Discharge Tube. | Crookes |
| 58a. | Mechanical Force of Cathode Rays. Wheel Caused to Rotate. | Crookes |
| 59. | Action of Magnet upon Cathode Rays in Discharge Tube. | Crookes |
| 60. | Mutual Repulsion of Cathode Rays in Discharge Tube. | Crookes |
| 61. | Heat of Phosphorescent Spot. | Crookes |
| 61a. | Theoretical Considerations of Thomson (Kelvin). | |
| 61b, page 46. | Velocity of Cathode Rays. | Thomson, J. J. |
| 61b, page 47. | Cathode Rays Charged with Negative Electricity. | Perrin |
| 61c, | Zeugen’s Photograph of Mt. Blanc Not Due to Cathode Rays. | |
| 62. | Phosphorescence of Particular Chemicals by Cathode Rays. | Goldstein |
| 63. | Spectrum of Post-phosphorescence of Discharge Tube Compared with that of Red-hot Metals. | Kirn |
| 63a. | Chemical Action on Photographic Plate by Cathode Rays Inside of Discharge Tube. | De Metz |
| 63b. | The Passage of Cathode Rays through Thin Metal Plates within the Discharge Tube (no. § 64). | Hertz |
| § 65, top of page 53. | Cathode Rays Outside of the Discharge Tube whose Exit is an Aluminum Window. A Glow Outside of the Window. | Lenard |
| 65., end of page 53. | Properties of Cathode Rays in Open Air. | Lenard |
| 66. | Phosphorescence by Cathode Rays Outside of the Discharge Tube. | Lenard |
| 66a. | Transmission Tested by Phosphorescence. | |
| 67. | The Aluminum Window a Diffuser of Cathode Rays. | Lenard |
| 68. | Transmission of External Cathode Rays through Aluminum and Thinly Blown Glass. | Lenard |
| 69. | Propagation of External Cathode Rays. Turbidity of Air. | Lenard |
| 70. | Photographic Action by External Cathode Rays and at Points beyond the Glow. No Other Chemical Power Probable. Shadows of Objects by Light and by External Cathode Rays Compared. No Heat Produced by External Cathode Rays. | Lenard |
| 71. | External Cathode Rays and the Electric Spark Distinguished. Aluminum Window Not a Secondary Cathode. | Lenard |
| 72. | Cathode Rays Propagated, but Not Generated, in the Highest Possible Vacuum. Air Less Turbid when Rarefied. | Lenard |
| 72a. | Cathode Rays, while Traversing the Exhausted Observing Tube, Deflected by a Magnet. No Turbidity in a Very High Vacuum. | Lenard |
| 72b. | An Observing Tube for Receiving the Rays and Adapted to be Exhausted. | Lenard |
| 73. | Phenomena of Cathode Rays in an Observing Tube Containing Successively Different Gases at Different Pressures. Phosphorescent Screen Employed for Making the Test. | Lenard |
| 74. | Cause of the Glow Outside of the Aluminum Window. Glow Not Caused by External Cathode Rays. Sparks Drawn from the Aluminum Window. Transmission of External Cathode Rays Dependent Alone upon the Density of the Medium. | Lenard |
| 75. | External Cathode Rays of Different Kinds Variably Diffused. Theoretical Observations. | Lenard |
| 76. | Law of Propagation of External Cathode Rays. | Lenard |
| 77. | Charged Bodies Discharged by External Cathode Rays. Discharge at Greater Distances than Phosphorescence. Not Certain as to the Discharge Being Directly Due to Intermediate Air. | Lenard |
| 78. | Source, Propagation, and Direction of Cathode Rays. General Conclusions. | De Kowalskie |
| 79. | X-rays Uninfluenced by a Magnet. Source of X-rays Determined by Magnetic Transposition of Phosphorescent Spot. | Roentgen |
| 80. | Source of X-rays may be at Points within the Vacuum Space. Different Materials Radiate Different Quantities of X-rays. | Roentgen |
| 81. | Reflection of X-rays. | Roentgen |
| 82. | Examples of Penetrating Power of X-rays. | Roentgen |
| 83. | Permeability of Solids to X-rays Increases Much More Rapidly than the Thickness Decreases. | Roentgen |
| x84. | X-rays Characterized. Fluorescence and Chemical Action. | Roentgen |
| 85. | Non-refraction of X-rays Determined by Opaque and Other Prisms. Refraction, if Any, Exceedingly Slight. | Roentgen |
| 86. | Velocity of X-rays Inferred to be the Same in All Bodies. | Roentgen |
| 87. | Non-double Refraction Proved by Iceland Spar and Other Materials. | Roentgen and Mayer |
| 88. | Rectilinear Propagation of X-rays Indicated by Pin-hole Camera and Sharpness of Sciagraphs. | Roentgen |
| 89. | Interference Uncertain Because X-rays Tested were Weak. | Roentgen |
| 90. | Electrified Bodies, whether Conductors or Insulators, or Positive or Negative, Discharged by X-rays. Hydrogen, etc., as the Intermediate Agency. | Roentgen |
| 90a. | Application of Principle of Discharge by X-rays. | Roentgen |
| 90A, b, c, d. | Supplementary Experiments on Charge and Discharge by X-rays. | Minchin, Righi, Benoist, Hurmuzescu, and Borgmann |
| 91. | Focus Tube. | Roentgen, Shallenberger, et al. |
| 91a. | Tribute to the Tesla Apparatus. | Roentgen |
| 92. | X-rays and Longitudinal Vibrations. | Roentgen |
| 93. | Longitudinal Waves in Luminiferous Ether by Electrical Means Early Predicted by | Thomson (Kelvin) |
| 94. | Theory as to X-rays Being of a Different Order of Magnitude from those so far Known. | Schuster |
| 95. | Longitudinal Waves Exist in a Medium Containing Charged Ions. Theoretical. | Thomson, J. J. |
| 96. | Practical Application of X-rays Foreshadowed. | Boltzmann |
| 97. | The Sciascope. | Magie, Salvioni, et al. |
| 97a. | Electrified Bodies Discharged by Light of a Spark, and the Establishment of a Radical Discovery. | Hertz |
| 97b. | Above Results Confirmed and More Specific Tests. | Wiedemann and Ebert |
| 98. | Negatively Charged Bodies Discharged by Light. Discharge from Earth’s Surface Explained by Inference and Experiment. | Elster and Geitel |
| 99. | Relation between Light and Electricity. Cathode of Discharge Tube Acted upon by Polarized Light and Apparently Made a Conductor Because of the Discharging Effect. | Elster and Geitel |
| 99A to 99T. | Briefs Regarding Action between Electric Charge and Light. | Schuster, Righi, Stolstow, Branly, Borgmann, Mebius, et al. |
| 100. | Stereoscopic Sciagraphs. | Thomson, E. |
| 101. | Obtaining Manifold Sciagraphs Simultaneously upon Superposed Photographic Films and through Opaque Materials, and thus Indicating Relative Sensitiveness of Different Films to X-rays. Intensifying Process Applicable in Sciagraphy. Thick Films Appropriate. | Thomson, E. |
| xi101a. | Sciagraph Produced through 150 Sheets of Photographic Paper. | Lumière. |
| 102. | Discharge Tube Adapted for Both Unidirectional and Alternating Currents. | Thomson, E., and Swinton |
| 103. | X-rays. Opalescence and Diffusion. | Thomson, E., Pupin, and Lafay |
| 103a. | Diffusion and Reflection in Relation to Polish. | Imbert, et al. |
| 104. | Fluorometer. Fluorescing Power of Different Discharge Tubes Compared. | Thomson, E. |
| 105. | Modified Sciascope for Locating the Source and Direction of X-rays. Phosphorescence Not an Essential Accompaniment in Production of X-rays. | Thomson, E. |
| 106. | X-rays from Discharge Tube Excited by Wimshurst Machine. Full Details Given of the Electrical Features. | Rice, Pupin, and Morton |
| 107. | Source of X-rays Determined by Projection through a Small Hole upon Fluorescent Screen Adjustable to Different Positions. | Rice |
| 107a. | Use of Stops in Sciagraphy. | Leeds and Stokes |
| 107b. | X-rays from Two Phosphorescent Spots. | Macfarlane, Klink, Webb, Clark, Jones, and Morton |
| 108. | Source of X-rays Determined by Shadows of Short Tubes. | Stine |
| 109. | Instructions Concerning Electrical Apparatus for Generating X-rays. | Stine |
| 110. | Apparent Diffraction Really Due to Penumbral Shadows. | Stine |
| 110a. | Non-diffraction. | Perrin |
| 159a. | Non-Refraction | |
| 111. | Source of X-rays Tested by Interceptance of Assumed Rectilinear Rays from the Cathode. | Scribner and M’Berty |
| 112. | Source of X-rays on the Inner Surface of the Glass Tube Determined by Pin-hole Images. | Scribner and M’Berty, Perrin |
| 112a. | Anode Thought to be the Source. Cause of Error Suggested. | De Heen |
| 113. | Pin-hole Pictures by X-rays Compared with Pin-hole Images by Light to Determine the Source. X-rays Most Powerful when the Anode is the Part Struck by the Cathode Rays. | Lodge |
| 114. | Valuable Points Concerning Electrical Apparatus Employed. | Lodge |
| 115. | X-rays Equally Strong during Fatigue of Glass by Phosphorescence. | Lodge |
| 116. | Area Struck by Cathode Rays Only an Efficient Source when Positively Electrified. | Rowland, Carmichael, and Briggs |
| 117. | Transposition of Phosphorescent Spot and of Cathode Rays without a Magnet. | Salvioni, Elster, Geitel, and Tesla |
| 117a. | Molecular Sciagraphs in a Vacuum Tube. | Hammer and Fleming |
| 118. | X-rays Begin before Striæ End. | Edison and Thomson, E. |
| 119. | Reason why Thin Walls are Better than Thick. | Edison |
| 120. | To Prevent Puncture of Discharge Tube by Spark. | Edison |
| 121. | Variation of Vacuum by Discharge and by Rest. | Edison |
| xii122. | External Electrodes Cause Discharge through a Higher Vacuum than Internal. | Edison |
| 123. | Profuse Invisible Deposit from Aluminum Cathode. | Edison and Miller |
| 124. | Possible Application of X-rays. Fluorescent Lamp. | Edison and Ferranti |
| 124a. | Greater (?) Emission of X-rays by Easily Phosphorescent Materials. | Piltchikoff |
| 125. | Electrodes of Carborundum. | Edison |
| 126. | Chemical Decomposition of the Glass of the Discharge Tube Detected by the Spectroscope. | Edison |
| 127. | Sciagraphs. Duration of Exposure Dependent upon Distances. | Edison |
| 128. | Differences between X-rays and Light Illustrated by Different Photographic Plates. Times of Exposure. | Edison, Frost, Chappin, Imbert, Bertin-Sans, and Meslin |
| 128a. | Georges Meslins insured a reduction of time for taking sciagraphs by the deflection of the cathode rays by means of a magnetic field | |
| 129. | Size of Discharge Tube to Employ for Given Apparatus. | Edison |
| 130. | Preventing Puncture at the Phosphorescent Spot. | Edison |
| 131. | Instruction Regarding the Electrical Apparatus. | Edison and Pupin |
| 132. | Salts Fluorescent by X-rays. 1800 Chemicals Tested. | Edison |
| 133. | X-rays Apparently Passed around a Corner. Theoretical Consideration by Himself and Others. | Edison, Elihu Thomson, Anthony, et al. |
| 134. | Permeability of Different Substances to X-rays. A List of a Variety of Materials. | Edison and Terry |
| 134a. | Illustration of Penetrating Power of Light. | Hodges |
| 135. | Penetrating Power of X-rays Increased by Reduction of Temperature. Tube Immersed in Oil, and the Oil Vessel in Ice. X-rays Transmitted through Steel 1/8 in. Thick. | Edison |
| 136. | X-rays Not Obtainable from Other Sources than Discharge Tube. | Edison, Rowland, et al. |
| 137. | Kind of Electrical Apparatus for Operating Discharge Tube for Powerful X-rays. | Tesla and Shallenberger |
| 138. | How to Maintain the Phosphorescent Spot Cool. | Tesla |
| 139. | Expulsion of Material Particles through the Walls of a Discharge Tube. | Tesla |
| 139a. | Giving to X-rays the Property of Being Deflected by a Magnet. | Lafay and Lodge |
| 139b. | Penetration of Molecules into the Glass of the Discharge Tube. | Gouy |
| 140. | Vacuum Tubes Surrounded by a Violet Halo. | Tesla and Hammer |
| 141. | Anæsthetic Properties of X-rays. | Tesla and Edison |
| 142. and 142a. | Sciagraphs of Hair, Fur, etc., by X-rays. Pulsation of Heat detected. | Tesla, Morton, and Norton |
| 143. | Propagation of X-rays through Air to Distances of 60 ft. | Tesla |
| 144. | X-rays with Moderate Vacuum and High Potential. | Tesla |
| 145. | Detailed Construction and Use of Single Electrode Discharge Tubes for Generating X-rays. | Tesla |
| xiii146. | Percentage of Reflection. | Tesla and Rood |
| 146a. | Reflected and Transmitted Rays Compared. Practical Application of Reflection in Sciagraphy. Analogy between Reflecting Power of Metals and their Position in the Electro-positive Series. | Tesla |
| 147. | Discharge Tube Immersed in Oil. Rays Transmitted through Iron, Copper, and Brass, 1/4 in. Thick. | Tesla |
| 148. | Bodies Not Made Conductors when Struck by X-rays. | Tesla |
| 149. | Non-conductors Made Conductors by a Current. | Appleyard |
| 149a. | Appleyard’s Experiment. Non-conductors Made Conductors by Current. | |
| 150. | Electrical Resistance of Bodies Lowered by the Action of Electro-magnetic Waves. | Minchin |
| 151. | Sciagraphic Plates Combined with Fluorescent Salts. | Pupin, Swinton, and Henry. |
| 152. | Penetrating Power of X-rays Varies with the Vacuum. | Thompson, S. P. |
| 153. | Reduction of Contact Potential of Metals by X-rays. | Murray |
| 154. | Transparencies of Objects to X-rays Not Influenced by the Color. Detected by Simultaneous Photographic Impressions. | Nodon, Lumière, Bleunard, and Labesse |
| 155. | Chlorine, Iodine, Sulphur, and Phosphorus Combined with Organic Materials Increase Opacity. | Meslans, Bleunard, and Labesse |
| 156. | Application of X-rays to Distinguish Diamonds and Jet from Imitations. | Buquet, Gascard, and Thompson, S. P. |
| 157. | Inactive Discharge Tubes Made Luminous by X-rays. | Dufour |
| 158. | Non-refraction in a Vacuum. | Beaulard |
| 159. | Bas-relief Sciagraphs by X-rays. | Carpentier and Miller |
| 160. | Transparency of Eye Determined by Sciagraph of Bullet Therein. | Wuillomenet |
| 161. | Mineral Substances Detected in Vegetable and Animal Products. | Ranwez |
| 162. | Hertz Waves and Roentgen Rays Not Identical. | Errera |
| 163. | Non-mechanical Action by X-rays Determined by the Radiometer. | Gossart |
| 164. | X-rays within Discharge Tube. | Battelli |
| 165. | Combined Camera and Sciascope. | Bleyer |
| 166. | Non-polarization of X-rays. | Thompson, S. P., Macintyre |
| 167. | Diffuse Reflection. Dust Figures Indirectly by X-rays. | Thompson, S. P. |
| 168. | Continuation of Experiments in § 113. | Lodge |
| 169. | Thermopile Inert to X-rays. | Porter |
| 170. | Non-diffraction of X-rays. | Magie |
| 171. | Resistance of Selenium Reduced by X-rays. | Giltay and Haga |
| 200. | Needle Located by X-rays and then Removed. | Hogarth |
| 201. | Needle Located at Scalpel by X-rays and then Removed. | Savary |
| 202. | Diagnosis with Fluorescent Screen. | Renton and Somerville |
| 203. | Bullet Located by Five Sciagraphs. | Miller |
| 204. | Bones in Apposition Discovered by X-rays and afterward Remedied by Operation. Other Cases. | Miller |
| 204a. | Necrosis. | Miller |
| 205. | Application of X-rays in Dentistry. | Morton |
| 206. | Elements of the Thorax. | Morton |
| 207. | A Colles’ Fracture Detected by X-rays. | Morton |
| 208. | Motions of Liver, Outlines of Spleen, and Tuberculosis Indicated. | Morton and Williams |
| 209. | Osteomyelitis distinguished from Periostitis. | Lannelongue, Barthelemy, and Oudin. |
| 210. | Concluding Miscellaneous Experiments Relating to Similar Applications of X-rays. | Ashhurst, Packard, Müller, Keen, and Morton, T. G. |
| Theoretical Considerations, Arguments, and Kindred Radiations. | Anthony |
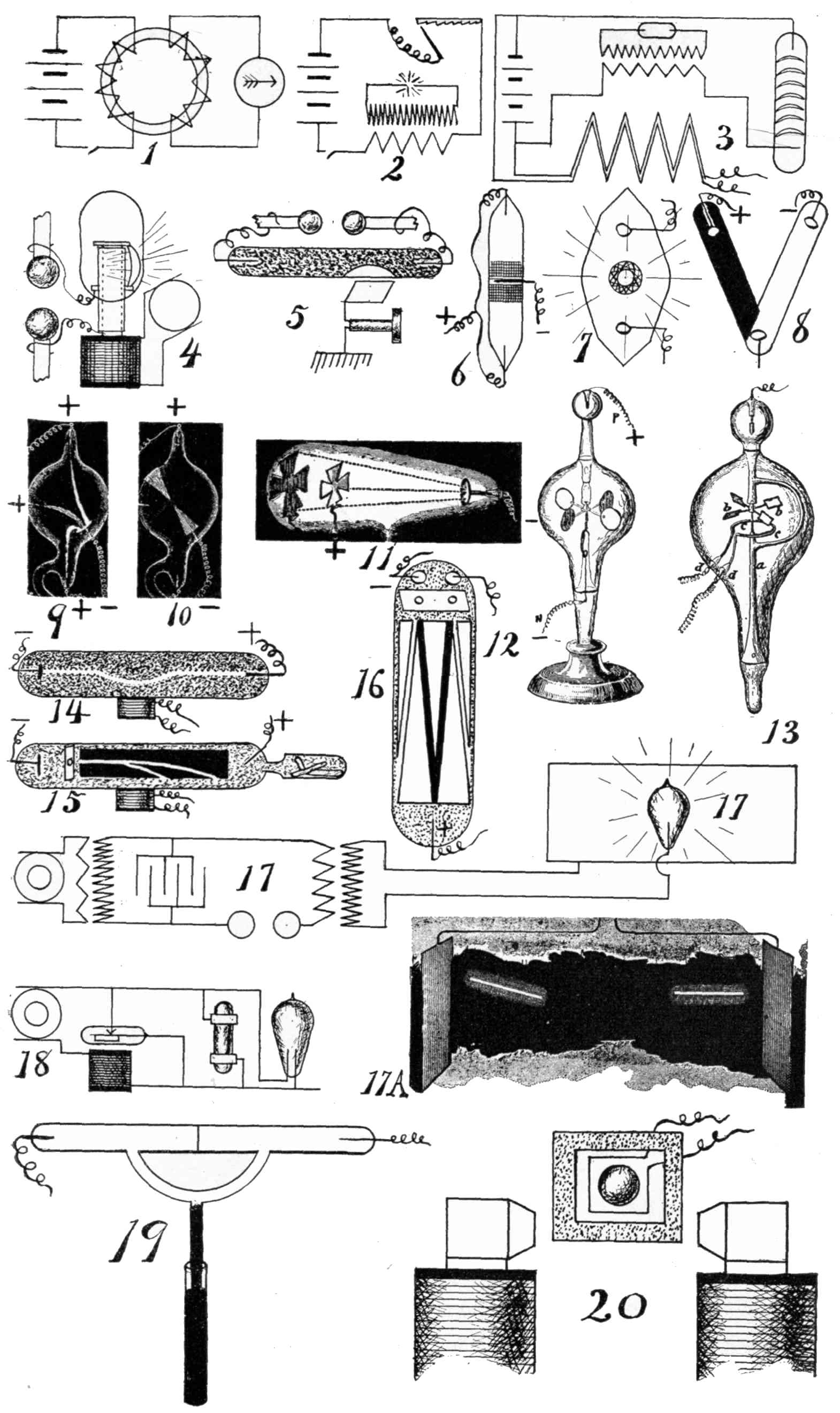
The new form of energy, for which there are two names—to wit, the Roentgen ray and the X-ray—is radiated from a highly exhausted discharge tube, which may be energized by an induction coil or other suitable electrical apparatus, such as a Holtz or a Wimshurst electrical machine. § 106. The principle underlying the construction of the usual induction (or Ruhmkorff) coil is disclosed in the subject-matter of § § 1, 2, and 3, and is represented in diagram in Figs. 1 and 2 on page 17. It would be well for the amateur or general scientific reader to study these sections carefully, for then he will have all the knowledge that is necessary for understanding the apparatus by which the discharge tube is energized. Of course, he will not comprehend the various mechanical details, nor the many electrical and mathematical relations existing in connection with an induction coil, but he will gain sufficient knowledge to appreciate what is intended when such a device is referred to here and there throughout the book. Since the time of Faraday, Page, and Fizeau induction coils of very large dimensions have been constructed, but none of them probably ever exceeded that built by Spottiswoode, during or about 1875, which was so powerful as to produce between the two electric terminals, in open air, a spark of 42 in. in the secondary current with only 30 small galvanic cells of the Grove type in the primary circuit. The cells are seldom used in this connection at the present time, the same being replaced by the dynamo, and the current being conveniently obtained from the regular incandescent-lamp circuit which may be found in almost any city. Those, therefore, who intend to become better acquainted with the details of the electrical apparatus should study in conjunction with this book some elementary treatise relating particularly to dynamos and electric currents.
xviThe essential element in connection with the generation of X-rays is not the coil nor the dynamo, but the electric discharge, especially when occurring within a rarefied atmosphere, provided within a glass bulb, called the discharge tube throughout the book, but which has usually been called by different names, for example, the receiver of an air pump, or a Geissler tube, when the air is not very highly exhausted, or a Crookes tube (see picture at § 123) when the vacuum is definitely much higher by way of contrast. It has also been called a Hittorff tube, the Lenard tube, and by several other names, according to its peculiar characteristics.
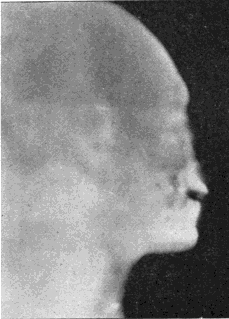
Fig. 1.—Head.

Fig. 2.—Broken Arm, Overlapping.
(Due to defective setting.)
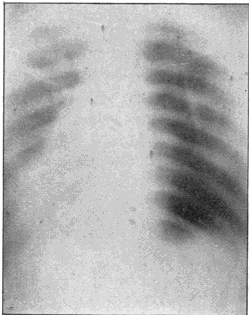
Fig. 3.—Ribs.
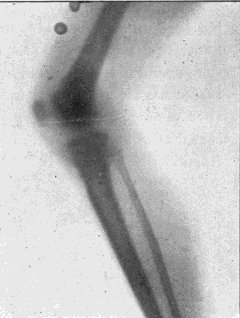
Fig. 4.—Knee, Knickerbocker Buttons, Bullet in Femur.
For those who are not acquainted with the nature of the electric charge and discharge, nor with the peculiar and exceedingly interesting phenomena which various investigators have discovered from time to time, nor with the variety of effects according to the nature and the pressure of the atmosphere within the glass bulb, it is exceedingly difficult to understand with any degree of satisfaction the properties, principles, laws, theories, and manner of application of cathode and X-rays. Consequently, the greater part of the book treats of the electric charge and discharge in conjunction with certain kindred phenomena. Primarily, the meaning of the electric discharge may be derived by referring to Fig. 2, page 17, where there is shown an electric spark, indicated by radial lines between the terminals of a fine wire forming the long and fine coil or secondary circuit. Imagine that the wires are at great distances apart. Let them be brought closer and closer together. By suitable tests it will be found, for example, that no current passes through the wire, but when the points are brought sufficiently close together a spark will occur between the two terminals. § 2. Sometimes instead of what is understood as a spark, a brush or glow takes place (§ § 10 and 11), and in fact a numerous variety of effects occur, a general name for all being conveniently termed an electric discharge. Even if no sudden discharge takes place, yet, as when the terminals are far apart, there may be a charge or a tendency, or, as it is technically called, a difference of potential, between the two electrodes, one of which is the cathode and the other the anode. This is comparable to a weight upon one’s hand, tending continually to fall, and always exerting a pressure, and it will fall when the hand xviiis suddenly removed. This is in the nature more of an analogy than of an exact correspondence. A discharge through open air, while adapted to produce a great many curious as well as useful effects, does not act as a generator of X-rays. § 136. Another class of phenomena is obtainable by exhausting the air to a certain extent from a discharge tube, thereby obtaining what is usually called a low vacuum. Such bulbs have been called Geissler tubes. Neither can X-rays be generated therefrom to any practicable extent, but only feebly if at all. § 118. Hittorff, Varley (§ 61a), Crookes (§ § 53 to 61, inclusive), were the first to discover and study the different phenomena that are obtained by diminishing the pressure within the discharge tube to a decrement of several thousand millionths of an atmosphere. This will explain why so many allusions have been made to the Crookes tube, for when the electric discharge is caused to take place in such a high vacuum X-rays are propagated in full strength.
Upon the first announcement of the discovery, electricians, eminent and otherwise, were of one mind in assuming the possibility of obtaining Roentgen rays from other sources than that of the highly evacuated discharge tube. Instead of speculating and theorizing, hosts of crucial tests were instituted, resulting negatively, and it is now safe to conclude that the electric discharge is the only primary source, and it is reasonably safe to assert that the discharge must take place within a highly evacuated enclosure.
The next stage of exhaustion, of no advantage to be considered, is that at which no discharge takes place (§ 25), and neither are any Roentgen rays propagated therefrom.
1. Faraday’s Experiment, 1831. Secondary Current by Induction. Experimental Researches, Proc. Royal. So. 1841.—In brief, the experiment involved the elements illustrated in the accompanying diagram, Fig. 1, p. 17; a ring made of iron; upon the ring, two coils of copper wire, suitably insulated from each other and from the iron; a galvanometer included in circuit with one coil, and an electric battery of ten cells placed in circuit with the other coil. He found that upon breaking or completing connection with the battery, the needle was powerfully deflected. Without entering into further detail, it is important, however, to notice that he did not perform any experiments tending to establish the principle of increase of E. M. F. by making the very slight change now known to be necessary. § 2.
2. Page’s Experiment, 1838. Electric Spark by Induced Current. Pynchon, p. 427. Dr. Page performed an experiment in which the primary coil was but a few feet in length, while the secondary coil was 320 ft. He included, in the primary circuit, only a few cells of battery. The manner in which he first caused rapid interruptions of the circuit of the primary coil was by the use of what may be called a coarse file, Fig. 2, p. 17. He discovered that the E. M. F. during the rapid interruption was so much increased over that of the small battery, that an electric spark would pass between the secondary terminals without first bringing them into contact with each other. § 6. The result of these experiments was not only the generation of a current of high E. M. F. from a generator of low E. M. F., but also a current of great quantity as compared with currents obtained from frictional and influence machines, whose complete history is found in Mascart’s work on Electricity.
3. Fizeau’s Experiment. Spark in Secondary Increased by Condenser in Primary, 1853. Pynchon, p. 456.—He connected the plates of a condenser respectively to the terminals of an automatic circuit breaker in the primary circuit, and noticed that the sparks between the two terminals of the interrupter 2produced by the self-induced current were greatly diminished, while those of the secondary coil were about double in length. Since that time it has been universally customary to equip induction coils with condensers in like manner.
4. Vincentini’s Experiment. Condition of a Gas Around a Live Wire. Nuovo Cimento, Vol. XXXVI., No. 3. Nature, Lon., March 28, ’95, p. 514. The Elect., Lon., Feb. 8, ’95, p. 433. G. Vincentini and M. Cinelli found that the molecules of a gas at and near the surface of a platinum wire, rendered incandescent by a current, are electrified, and that with hydrogen their potential is about .025 volt above the mean potential of the wire. With air and carbonic acid gas the increment is about 1 volt. The apparatus, Fig. II., consists essentially of means for passing a current along a platinum wire, a bulb for preventing draughts, and an electrometer having a platinum disc electrode that could be adjusted to different positions. It was noticeable that the electrification did not reach a maximum instantaneously upon closing the current through the wire, but the time was less at points below the wire than above.
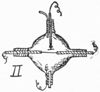
II
5. Henry’s Experiment. Magnetizing Radiations from an Electric Spark. Proc. Inter. Elect. Cong., 1893, p. 119. Preece alluded to Prof. Henry’s original experiment illustrating the action of an electric discharge § 2 at a distance. He placed a needle in the cellar. Disruptive discharges of a Leyden jar at 30 ft. distant, in an upper room, produced a magnetic effect upon the needle.
6. Faraday’s Experiment. Arc Maintained by Certain Metallic Electrodes at Low Voltage. Experimental Researches. Phil. Trans., Se. IX., Dec., 1894. § 107. to 1078. The generator employed in this experiment consisted of a few cells of a chemical battery, and he obtained, what he called, a voltaic spark. He observed that when the two terminals touched each other, a burning took place and an appearance as if the spark were passing on making the contact, the terminals being pointed and formed of metal. When mercury was the terminal, the luminosity of the spark was much greater than with platinum or gold, although the same quantity of current passed in both cases. He attributed the difference to a greater amount of combustion in the case of mercury, than in those of gold and platinum. He obtained almost a continuous spark by bringing down a pointed copper wire to the surface of mercury and withdrawing it slightly. Wheatstone, in 1835, analysed the light of 3sparks, and found them to be so characteristic that by means of the prism and the spectra formed, the metal could be known.

III
7. Wurts’s Experiment. Non-arcing metals at high voltage. Trans. Amer. Inst. Elect. Eng. March 15, 1892. Ann. Chem. Phar. Sup. VII, 354 and VIII, 133. Chem. News, VII, 70; X, 59, and XXXII, 21, 129.—Mendelejeff and Meyer discovered that chemical elements occur in natural groups by a principle which they termed the periodic law. One of these groups includes zinc, cadmium, mercury and magnesium; and another group, antimony, bismuth, phosphorus and arsenic. Alex. J. Wurts, of the Westinghouse Electric Co. found that the metals of these groups are non-arcing, by which he means that with an alternating current dynamo of a thousand or more volts, and with the said metals as electrodes in the air only just escaping each other, it is impossible to maintain an arc as in the case of an ordinary arc lamp having carbon electrodes or in a lightning arrester usually having copper electrodes. He suggested and theorized that certain chemical reactions served to explain the phenomena. With low voltage—as 500, the arc was maintained between all metals. § 6. A two pole lightning arrester is shown in Fig. III The arc formed, ceased instantly. One of the best metals for practical use is an alloy of 1/2 zinc and 1/2 antimony, or any metal electroplated with a non-arcing metal. Freedman observed a critical point with electrodes of brass. The current was gradually reduced until the arc became like the discharge of a Holtz machine whose condensers have been disconnected. See Elect. Power, N.Y., Feb. 1896, p. 119.
8. Wheatstone’s Experiment. Duration of Spark. Phil. Tran. 1834.—The short duration of an electric spark produced by a single disruptive discharge is easily made apparent by a rapidly rotating disc, having radial sectional areas of different colors. With reflected sunlight, the colors seem to blend into one tint upon the principle of the persistence of vision; (See Swain’s experiment. Trans. R. So. Edin. ’49 and ’61.); but when viewed by the flash of a spark, the colors are seen as distinctly separated as if the disc were at rest. By calculation, based directly upon a series of experiments, he found the duration of the spark to be about .000042 sec. It was discovered also, by the rotating mirror, that the apparently single spark was composed of several following each other in quick succession, and he concluded that the current during the discharge was intermittent. He considered each of the divisions of the spark as an electric 4discharge. Prof. Nichols, of Cornell University, and McKittrick obtained curves indicating the variation of E. M. F. during the existence of a spark. Trans. Amer. Inst. Elect. Eng. May 20, ’96.
8a. Feddersen, who used a Leyden jar, modified the experiment by having high resistances in the circuit through which the charge was effected. The duration of the spark was found to be increased. In one experiment, he employed a slender column of water as the resistance, 9 mm. in length. The spark endured .0014 second. With a tube of water 180 mm. the duration was .0183 second. He noticed also that the duration increases directly with the striking distance and with the electrical dimensions of the electrical generator. By varying the resistance of the circuit, he found as it became less, the discharge was intermittent, when further reduced, continuous, (difficult to obtain) § 11 and when very small, oscillatory—i.e., alternately in opposite directions.
9. Faraday’s Experiment. Brush discharge sound. Phil. Trans. Jan. 1837. Se. XII.—The brush discharge was caused to occur, in his experiments, generally from a small ball about .7 of an inch in diameter, at the end of a long brass rod, acting as the anode. With smaller balls he noticed that the pitch of the sound produced was so much higher as to produce a distinct musical note, and he suggested that the note could be employed as a means of counting the number of intermissions per second. See Mayer’s book on “Sound” § 77, on measuring number of vibrations in a musical note.
9a. Upon bringing the hand toward the brush the pitch increased. § 49. With still smaller balls and points, in which case the brush could hardly be distinguishable, the sound was not heard. He alluded to the rotating mirror of Wheatstone as becoming not only useful but necessary at this stage. He considered the brush as the form of discharge between the contact and the air or else some other non or semi-conductor, but generally between the conductor and the walls of the room or other objects which are nearest the electrodes, the air acting as the dielectric. One experiment, he performed with hydrochloric acid led him to believe that that particular gas permitted of a dark or invisible discharge. Sometimes the air was electrically charged § 4 to a less distance than the length of the brush or light.
10. Brush in Different Gases. Striae Cathode Brushes. In the air, at the ordinary pressure he found the color to be “purple;” when rarefied still more purple, and then approaching to rose; in oxygen, at the ordinary pressure, a dull white; when rarefied, “purple;” and with nitrogen, the color was particularly 5easily obtained at the anode, and when nitrogen was rarefied the effect was magnificent. The quantity of light was greater than with any other gas that he tried. Hydrogen, as to its effect, fell between nitrogen and oxygen. The color was greenish grey at the ordinary pressure and also at great rarity. The striae were very fine in form and distinctness, pale in color and velvety in appearance, but not as beautiful as those in hydrogen. With coal gas, the brushes were not easily produced. They were short and strong and generally green, and more like an ordinary spark. The light was poor and rather grey. Also in carbonic acid gas the brush was crudely formed at the ordinary pressure as to the size, light and color. The tendency of the discharge in this case was always towards the formation of the spark as distinguished from the brush. When rarefied, the light was weak, but the brush was better in form and greenish to purple, varying with the pressure and other circumstances. As to hydrochloric acid, it was difficult to obtain a brush at the ordinary pressure. He tried all kinds of rods, balls and points, and while carrying on all these experiments he kept two other electrodes out in the air for comparison, and while he could not obtain any satisfactory brush in the hydrochloric acid gas, there were simultaneously beautiful brushes in the air. In the rarefied gas, he obtained striae of a blue color.
He compared the appearances also of the anode and cathode brushes in different gases at different pressures. He noticed that in air, the superiority of the anode brush was not very marked (§ 41 at end.) In nitrogen, this superiority was greater yet. A line of theory ran through Faraday’s mind in connection with all these experiments, whereby he held that there is “A direct relation of the electric forces with the molecules of the matter concerned in the action.” § 47. He made a practical application of the principles in the explanation of lightning, because nitrogen gas forms 4/5 of the atmosphere, and as the discharge takes place therein so easily.
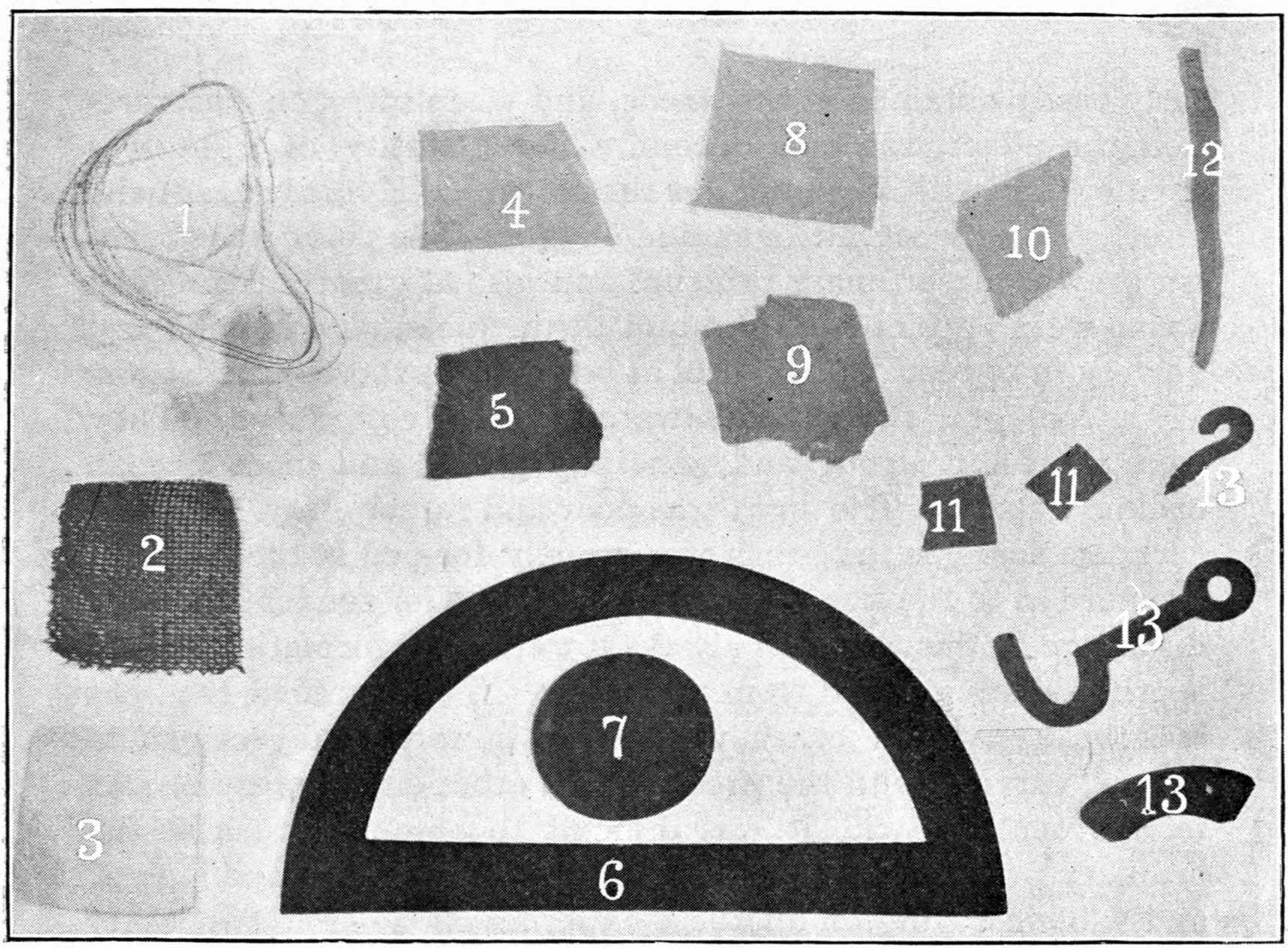
From Magnetographs by Prof. McKay. p. 25.
1. Platinum wire.
2. Copper gauze.
3. Iron gauze.
4. Tinfoil.
5. Gold-foil.
6. Brass protractor.
7. Silver coin.
8. Platinum-foil.
9. Brass.
10. Lead-foil.
11. Aluminum.
12. Magnesium ribbon.
13. Copper objects.
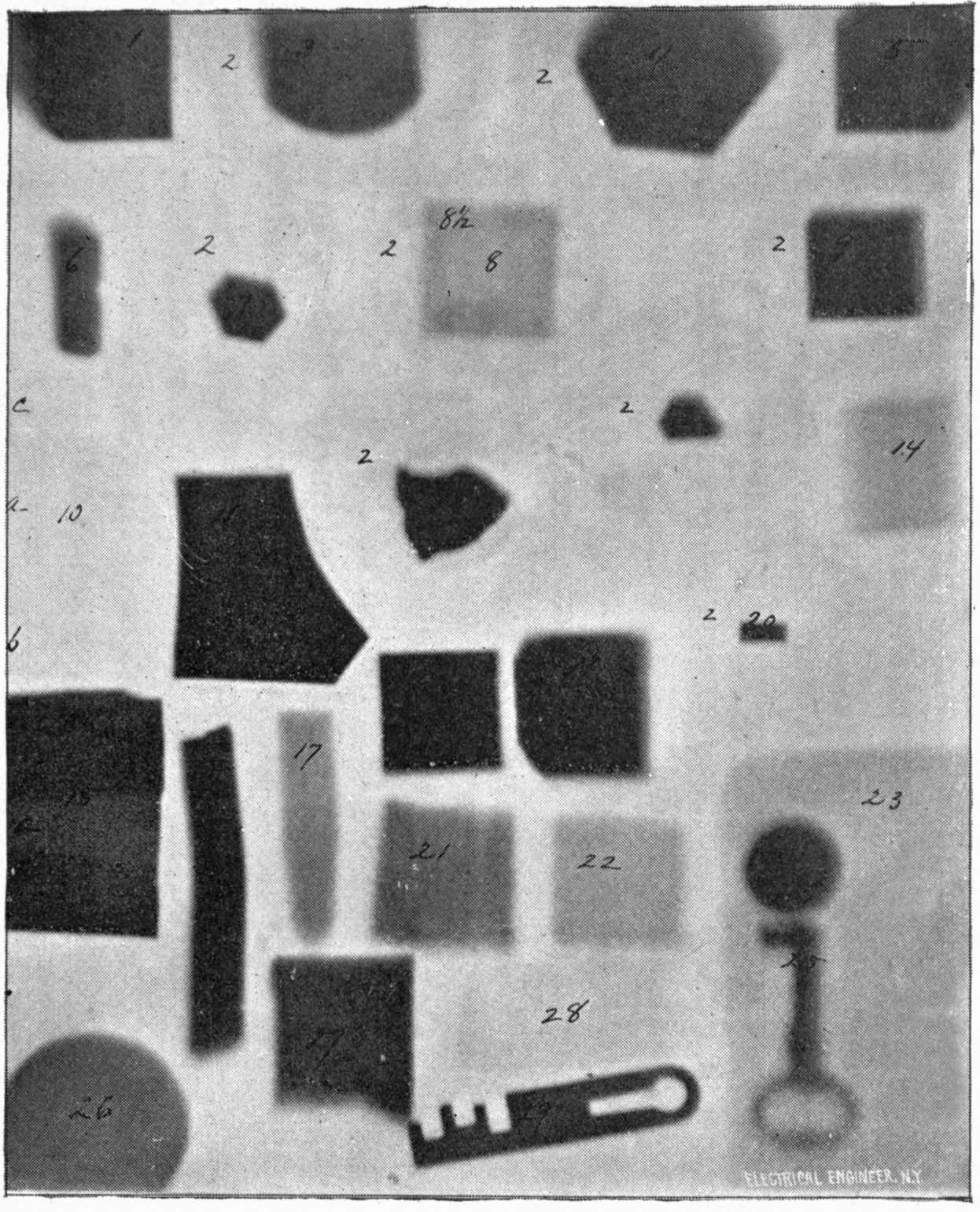
From Sciagraph of Various Objects. p. 130.
By Prof. Terry, U. S. Naval Academy.
11. Glow by Discharge. Glow Changed to Spark. Motion of Air. Continuous Discharge During Glow. The glow was most easily obtained in rarefied air. The electrodes were of metal rods about .2 of an inch in diameter. He also obtained a glow in the open air by means of one or both of the small rods. He noticed some peculiarities of the glow. In the first place, it occurred in all gases and slightly in oil of turpentine. It was accompanied by a motion of the gas, either directly from the light or towards it. He was unable to analyze the glow into visible elementary intermittent discharges, nor could he obtain 7any evidence of such an intermittent action, § 8a. No sound was produced even in open air. § 9. He was able to change the brush into a glow by aiding the formation of a current of air at the extremity of the rod. He also changed the glow into a brush by a current of air, or by influencing the inductive action near the glow. The presentation of a sharp point assisted in sustaining or sometimes even in producing the glow; so also did rarefaction of the air. The condensation of the air, or the approach of a large surface tended to change the glow into a brush, and sometimes into a spark. Greasing the end of the wire caused the glow to change into a brush.
12. Lullin’s Experiment. Spark. Penetrating Power. Passage Through Solids. Encyclo. Brit. Article Electricity. He placed a piece of cardboard between two electrodes and discovered that a spark penetrated the material and left a hole with burnt edges. When the electrodes were not exactly opposite each other, the perforation occurred in the neighborhood of the negative pole. Later experiments have shown that a glass plate, 5 or 6 cm. in thickness, can be punctured by the spark of a large induction coil. The plate should be large enough to prevent the spark from going around the edges. The spark is inclined, also, to spread over the surface of the glass instead of piercing it, § 24. Glass has been cracked by the spark in some experiments.
13. Fage’s Experiment. Spark. Penetrating Glass. Holes Close Together. Practical Application. La Nature, 1879. Nature, Dec. 26, 1879, p. 189. The length of the spark from the secondary coil in air was 12 cm. One terminal of the secondary passed through an ebonite plate (18 cm. × 12) and touched the glass. Olive oil was spread around said terminal (§ 11 at end), and served to insulate the same. Oil dielectric in this connection originally employed at least prior to 1870. Remembered by Prof. Anthony as far back as 1872, who often performed the experiment according to instructions contained in a publication. The other terminal of the secondary coil was brought against the glass opposite the first terminal. The spark was then passed and the glass perforated, § 12. By pushing the glass along to successive positions and passing the spark at each movement, holes could be made very close together. In Nature, of 1896, the author noticed that certain manufacturers were introducing glass perforated with invisible holes to be used for windows as a means of ventilation without strong draughts. Perhaps the fine holes were made by means of the electric spark.
814. Knochenhaurer’s Experiments. Conducting Power of Gas. Spark. Penetrating Power. Relation of E. M. F. to Pressure of Gas. 1834. Pogg. Ann., Vol. LVII., and Gordon, Vol. II. Boltzmann’s experiment (Pogg. Ann., CLV., ’75), and calculation indicated that a gas at ordinary pressure and temperature must have a specific resistance at least 1026 times that of copper. Pogg. Ann., CLV., ’75. Sir William Thomson (Kelvin) confirmed this limit for steam, and Maxwell the same for mercury and sodium vapor, steam and air. From Maxwell’s MSS. Herwig was not sure but that the Bunsen burner flame and mercury vapor conducted. He allowed for the conductivity of the walls of the glass container. Braun treated of the conductivity of flames. Pogg. Ann., ’75.
14a. Varley found that 323 Daniel cells only just initiated a current through a hydrogen Geissler tube, and only 308 cells continued the current after once started. Knochenhaurer found that Harris’ (Phil. Trans., 1834) law did not hold exactly true, and that the ratio between the E. M. F. and the air pressure becomes greater and greater as the pressure becomes less and less. Harris thought the ratio was constant. The limits of his pressures were from 3 to 27.04 inches of mercury. Stated in other words, his results were the same as those of Harris and Masson (Ann. de Chimie, XXX., 3rd Se.), except that a small constant quantity should be added. § 16.
15. Gordon’s Experiment. Dust Particles Hasten Discharge. Gordon, Vol. II. Other experimenters had investigated the phenomena of the electric spark with different densities of the dielectric by a spark produced by a frictional or an influence machine, or, in a few cases, by powerful batteries without coils, while Gordon claims to be the first to carry out these experiments with an induction coil. He observed that when the discharging limit was nearly reached, small circumstances, such as a grain of dust or a rusting of the terminal by a former discharge, would cause the discharge to take place at a lower E. M. F., which should be allowed for.
16. Kelvin’s Experiment. Proc. R. So., 1860. Encyclo. Brit., Art. Elect. He used as the terminals, two plates. One of them was perfectly plane, while the other had a curvature of a very long radius. The object of this arrangement was to obtain a definite length of spark for each discharge. The plates were gradually moved away until the spark would no longer pass, and the reading of the distance was noted. The law which he found cannot well be expressed in the form of a rule or principle, because it is of a rather intricate nature, but a discovery 9resulted, namely in the case where the distance was greater, the dielectric strength was smaller for respective distances of .00254 and .535 cm. Many theoretical considerations in reference to this matter have been presented, notably that of Maxwell in his treatise on Electricity and Magnetism, Vol. I.
17. Cailletet’s Experiment. Spark. Penetrating Power. High Pressures. Increased Dielectric Strength. Mascart, Vol. I. He experimented with dry gas up as high as pressures of 700 lbs. per sq. inch. He found that the dielectric strength continues to increase with increase of pressure. He used about 15 volts in the primary and a powerful induction coil. The dielectric strength was so great that at the maximum pressure named above, the spark would not pass between the electrodes when only .05 mm. apart. § 25 and 11, near end.
18. Faraday’s Experiment. Discharges in Different Chemical Gases Variably Resisted. Exper. Res. Phil. Trans., Se. XII., Jan. ’36. Faraday passed on from the consideration of the effect of pressure, temperature, etc., and wondered whether there would be any difference in the law according to what gas was used. He arranged apparatus so that he could know, with air as a standard, whether another gas had a greater or less dielectric power. (Cavendish before him had noticed a difference.) He tabulated the results. They exhibited the following facts, namely that gas, when employed as dielectrics, depend for their power upon their chemical nature. § 10. Hydrochloric acid gas was found to have three times the dielectric strength of hydrogen, and more than twice that of oxygen, nitrogen or air; therefore the law did not follow that of specific gravities nor atomic weights. See also De la Rue, Proc. Royal So., XXVI., p. 227.
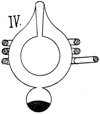
19. Thomson’s Experiments. Gas as a Conductor. Visible Indication by Discharge. Nature, Lon., Aug. 23, ’94, p. 409; Jan. 31, ’95, p. 332, and other references cited below. Lec. Royal Inst. Proc. Brit. Asso., Aug. 16, ’94. In making comparisons, things of like nature should be considered. Take, for example, gas at .01 m. The number of molecules in such a rarefied atmosphere is comparatively small, while in an electrolyte there are molecules sufficient in number to produce 15,000 lbs. of pressure, if imagined in the gaseous state within the same space. By an experiment and rough calculation, Prof. J. J. Thomson, F.R.S., calculated that the conductivity of a gas estimated per molecule is about 10 million times that of an electrolyte, for example, sulphuric acid. § 14. This is greater than the molecular conductivity of the best conducting metals. The experiment which is illustrated in Fig. IV. was a second experiment which 10did not serve as a basis for calculation, but exhibited very strikingly to the eye that gases having different pressures have different conductivities. For this apparatus he had two concentric bulbs, as indicated, one being contained within the other. The inner one had air rarefied to the luminous point. The outer one had a vacuum as high as it was practical to make it, and contained in a projection a drop of mercury, which, when heated, would gradually increase the pressure. Two Leyden jars were employed, and their outer coatings were connected to the coil which is seen surrounding the outer bulb, and the inner coatings were connected to the coils of a Wimshurst machine. The operation was as follows: When the mercury was cold, that is, with a high vacuum in the outer compartment, a bright discharge passed through the inner bulb, while the outer bulb was dark. When the mercury was heated, the outer bulb was bright, and the inner one was almost dark. By well-known principles of conductors and non-conductors, the operation was explained by Prof. Thomson, who assumed that the gas in the outer bulb is a conductor; then, at each spark will the alternating current in the coil induce currents of an opposite direction in the gas, which will become luminous, as occurred when the mercury was heated. The currents circulating in the gas act as a shield to the induction of the currents in the inner bulb. However, with the vacuum exceedingly high in the outer bulb, the air therein being a non-conductor comparatively, or for the given E. M. F., does not prevent the discharge through the inner bulb, which becomes, therefore, luminous. He next compared the dielectric power of a gas, a liquid and a solid. He found that the E. M. F. had to be raised, in order to produce the discharge,—higher in the liquid than in the gas, and higher in the solid than in the fluid. § 12.
IV.
20. Boltzmann, Gibson, Barclay, Hopkinson and Gladstone’s Experiments. Square Root of the Dielectric Capacity Equal to the Refractive Index. Phil. Trans., 1871, p. 573. Maxwell, Vol. II., § 788. Maxwell has argued elaborately upon results of some of the above experimenters upon the theory that the luminiferous ether is the medium for transmission of electricity, light and magnetism; therefore he predicted that the relation stated in the title above should exist. He acknowledged that the relation is sufficiently near a constant to show 11in connection with other results, especially those obtained, that his theory is probably correct.
21. PLÜCKER’s Experiment. Hermetically Sealed Vacuum Tube. Encycl. Brit., vol. 8, p. 64. Pogg. Ann., 1858, and vol. CXXXVI, 1869.—He engaged Geissler (according to Hittorf) to make a glass tube in which the platinum wire electrodes were sealed in the glass by fusion, as in the modern incandescent lamp. After the air was exhausted by a mechanical air pump through a capillary tube, the same was sealed with the flame of a spirit lamp. He thus established means whereby a practically permanent vacuum could be maintained within a glass bulb. Platinum expands by heat at about the same rate as glass: hence there is no tendency to crack and admit air.
22. Geissler’s Experiment. Luminosity of Vacuum Tubes by Friction. Increased by low temperature. Science Record, 1873.—By rubbing the vacuum tubes with an insulator—cat skin, silk, etc.—he observed that light was generated and that its color depended upon the particular gas forming the residual atmosphere. At a low temperature, the colors were more luminous. § 135. The best form of tube consisted of a spiral tube contained within another tube. A modified construction involved the introduction of mercury. By exhausting the air, and shaking the tube, the friction or motion of the mercury against the glass produced luminous effects according to the gas. Only chemically pure mercury would cause the light, which endured for an instant after the rubbing ceased. § 63.
23. Alvergniat’s Experiment. Luminosity of Vacuum Tubes by Friction and Discharges. Different Vacua Required. Sci. Rec., 1873, p. 111. Comptes Rendus, 1873.—To obtain luminosity by charging the tubes with the coil, it was necessary to increase the degree of the vacuum—but when this was done the rubbing of the tube would not cause light. The tube employed was 45 cm. in length, and contained a small quantity of silicic bromide. The atmospheric pressure within the tube for obtaining the glimmer by friction was 15 mm.
24. Steinmetz’s Experiment. Luminous Effects by Alternating Current and Solid Dielectrics. Trans. Amer. Inst. Elec. Eng., Feb. 21, ’93.—In carrying on experiments in the accurate measurement of dielectric strength, he noticed that upon placing mica between the electrodes, as is hereinafter set forth, a spark did not at first form, but that which he called a corona. He attributed the appearances to a condenser phenomenon, or at least he suggested this as an explanation. § 3. As soon as the corona reached the edge of the plate, the 12disruptive discharge took place, by means of the sparks passing over the edge of the dielectric. § 38. He employed an alternating current dynamo of about 50 volts and 1 h.p., frequency of 150 complete periods per second. The E. M. F. of the alternator was varied, by changing the exciting current, up to 90 volts. Step-up transformers were employed. With a difference of potential in the secondary of 830 volts, and a thickness of mica of 1.8 mm. and when the experiment was performed in a dark room a faint bluish glow appeared between the mica and the electrodes. At 970 volts the glow was brighter, while at 1560 volts the luminosity was visible in broad day-light, and kept on increasing with the increase of E. M. F. He modified the experiment by using mica of a thickness of 2.3 mcm. The difference of potential was 4.5 kilo-volts. In addition to the bluish glow, violet streams or creepers broke out and increased in number and length as the E. M. F. became greater, forming a kind of aurora around the electrodes and on both sides of the mica sheet. A loud hissing noise occurred. § 9. As soon as the corona reached the edges of the mica, the disruptive discharge occurred in the form of intensely white sparks and it was noticeable that the length of these sparks was 10 fold greater than could be obtained in the air at 17 kilo-volts. These sparks were so hot as to oxidize the mica, as apparent from the white marks remaining. The electrodes also became very hot, and the mica was contorted and finally broke down.
25. Morgan’s Experiment. No discharge in High Vacua. Wiedemann, vol. 2. Phil. Trans., 1875, vol. 75.—He was led to believe by an experiment, that when the vacuum is sufficiently perfect, no electromotive force could drive the spark from one terminal to the other, however close together they may be. § 18. Details of Morgan’s Experiments were as follows, given roughly in his own words:—A mercurial gauge about fifteen inches long, carefully and accurately boiled till every particle of air was expelled from the inside, was coated with tinfoil five inches down from its sealed end, and being inverted into mercury through a perforation in the brass cap which covered the mouth of the cistern, the whole was cemented together and the air was exhausted from the inside of the cistern, through a valve in the brass cap, which, producing a perfect vacuum in the gauge, formed an instrument peculiarly well adapted for experiments of this kind. Things being thus adjusted (a small wire having been previously fixed on the inside of the cistern, to form a communication between the brass cap and the mercury, into which the gauge was inverted), the coated end was applied to the conductor 13of an electrical machine, and notwithstanding every effort, neither the smallest ray of light nor the slightest charge could ever be procured in this exhausted gauge.
26. De La Rue and Müller’s Experiment. Constant Potential at the Terminals of a Discharge Tube. Phil. Trans., part 1, vol. 169, p. 55 and 155.—The apparatus consisted of an exhausted bulb, a chloride battery of 2400 cells and a large resistance adapted to be varied between very wide limits. The result was a constant potential at the electrodes of the bulb, during all the variations of the resistance. They concluded, therefore, that the discharge in highly rarefied gases is disruptive, the same as in air at ordinary pressure.
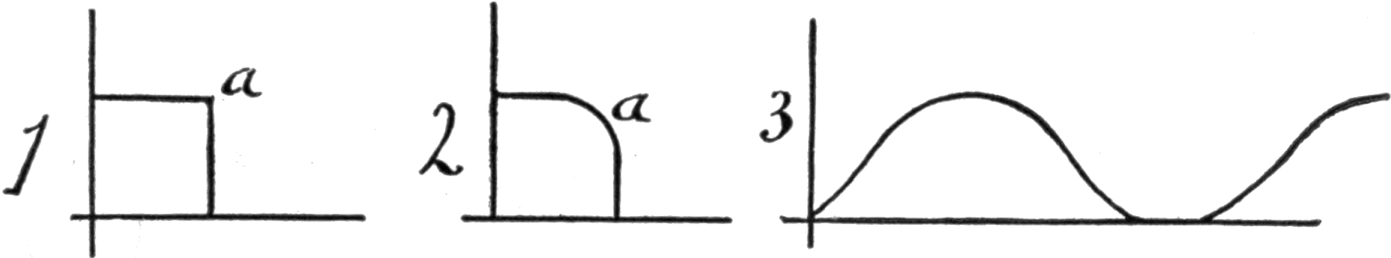
26a. Klingenberg’s Calculations. Direction of Discharge Tube Current in Secondary of Ruhmkorff Coil. Translated from the German, by Ludwig Gutmann. Extract of paper read by G. Klingenberg before the Electro-technischer Verein. It would naturally be inferred that an induction coil, the primary current of which is intermitted, and of one direction, would produce an alternating current in the secondary coil. The fact of the matter is, however, that a good induction coil will produce the sparking only in but one direction. § 41. The reason is the following: If the coil had no self-induction nor capacity, then the current impulses would be represented by a rectangle a, Fig. 1. On closing, the current would suddenly reach its maximum, which is determined by the terminal pressure and circuit resistance, and this current strength would be maintained as long as the circuit remained closed. On the opening of the circuit, the current would decrease just as suddenly; if not, the arc on opening of the circuit would oppose such sudden fall, therefore the corner will be slightly rounded at a, Fig. 2. The influence of self-induction, which we find in any coil, is the force that will tend to oppose any change in the current strength. Therefore, the self-induction will be the cause of a retardation of the minimum current. On the other hand, it increases the size of the spark on opening. Next a condenser is enclosed in the main circuit, so that the spool is closed through it at the moment the current is intercepted. If we assume, for simplicity sake, that the magnetization of the iron is proportional to the current strength, then the primary current curve represents at the same time, the curve of 14the rate of change of line of force in the magnetic field. The secondary E. M. F. is determined by e = n(dw/dt)t t; the rise then will have a smaller E. M. F. than at the fall, like Fig. 3, except that the curve representing the fall should be shown as more nearly perpendicular to the abscissa.
V
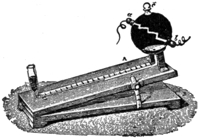
27. Kinnersley, Harris and Riess’s Experiments. Spark. Pressure Produced by. Ganot, § 790, et al. Encyclo. Brit. Art. Elect.—These experimenters passed a spark through air contained over mercury, so that if the pressure of the air were increased, the mercury would move along through a capillary tube, having a scale so that the amount could be represented to the eye, as in the cut. (Fig. V.) The experiments proved that when a spark passes through the air, the pressure is increased, and it was concluded in view of several experiments, that the spark being the source of an intense, but small amount of heat, expanded the air, thereby causing the pressure in a secondary manner, through the agency of heat. A spark as short as 2 mm. will produce a considerable pressure of the mercury. Riess performed an experiment also in causing the spark to pass through cardboard, and also through mica located within the air chamber. § 12. Other things being equal, the increase of temperature was less by using the solid material like mica or cards, than without. This illustrated that a part of the energy of the spark was converted into heat and a part into mechanical force, and explained why sound, § 24, is produced by a spark and by lightning.
VI
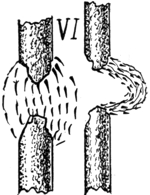
28. Davy, Bancalari and Quet’s Experiments. Electric Arc, Magnetism and Flame. Sound Produced. Practical Application of Electric Arc. Phil. Mag., 1801.—When the electric arc, for example between two carbon electrodes, occurs, in a powerful magnetic field, it is violently drawn to one side as first shown by Sir Humphry Davy, as if the wind were blowing it and sometimes it is broken into two parts. Fig. VI. Again a loud noise is produced. § 9. Without the magnet, the appearance is as at the left. With the energized magnet, the arc and light, as a whole, are as shown at the right.
29. De La Rive’s Experiment. Rotation of Luminous Effect by Magnet. Application to Explain Aurora Borealis. Phil Trans., vol. 137, 1847. Pynchon, p. 471. Ganot, Sect. 928.—An oval discharge tube was employed, having a highly exhausted atmosphere (for those days) of spirits of turpentine. A cylindrically shaped pole of a magnet extended into the bulb half way, Fig. 4, p. 17. The inner end of the magnetic pole formed one electrode of the tube, and the other electrode was a ring within the vacuum at the foot of the magnetic pole. A fountain of light extended from one end of the magnet pole to the other, and remained stationary, while the magnet was not energized; but the light was condensed into an arc and travelled around the magnet pole when a current was passed through the coils of the magnet. For similar action of magnet on a flexible and movable wire carrying a current, see experiments of Spottiswoode and Stokes, Proc. R. So., 1875. The aurora borealis rotates around the pole of the earth, and therefore, De La Rive thought that the phenomenon in his laboratory and in nature were but one and the same thing and different only in degree. He also extinguished an arc in open air by means of a powerful magnet.
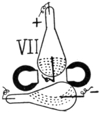
VII
30. Plücker and Hittorf’s Experiments. Action of Magnet on Cathode Column of Light. Pogg. Ann., 1858 and 1869. Plücker found that the magnet acts on the cathode light in a rarefied atmosphere in a different manner from that on the anode light. In the former the light follows the magnetic curves and strike the side of the bulb, according to position of the poles, see Fig. VII. “Where the discharge is perpendicular to the line of the poles, it is separated into two distinct parts, which can be referred to the different action exerted by the electro-magnet on the two extra currents produced in the discharge.” Ganot. § 925.
31. Thomson’s Experiment. A Discharge Retarded Across and Accelerated Along the Lines of Magnetic Force. Nature, Lon., Jan. 31, 1895, p. 333. Lect. Royal Inst.—Prof. J. J. Thomson, F. R. S., performed an experiment which illustrates that the electrical discharge is retarded in flowing across the lines of magnetic force and accelerated in flowing with or parallel to such lines. As illustrated in Fig. 20, p. 17, he employed a large electro-magnet adapted to be cut in and out of circuit. He had two air chambers, one a bulb, indicated by a circle, and the other a tube bent into a rectangle, indicated by the dotted square. Between these, was an adjustable coil having its terminals connected to the outside coatings of Leyden jars. When the discharge took place between the poles of the magnet, that is, in the direction of the lines of force, the discharge was helped along by the magnetic field, but when it took place across the bulb, that is, across the lines of force, the discharge was retarded. “The coil can be adjusted so that when the magnet is ‘off’ the discharge passes through the bulb, but not round the square tube; when, however, the magnet is ‘on,’ the discharge passes in the square tube but not in the bulb.”
Some Experiments prior to Lenard’s.
32. Thomson’s Experiment. Resistance Offered to Striae by a Thin Diaphragm. Lect. Royal Inst. Nature, Lon. Jan. 31, ’95, p. 333.—It has often been remarked that lightning always takes the easiest path. The same has been noticed with references to the artificial electric spark. Prof. J. J. Thomson, F.R.S. performed an experiment, which not only confirms this principle but does so in an emphatic manner, and proves it true in reference to the electric discharge in rarefied gases. He arranged a very thin platinum diaphragm so as to divide a Geissler tube into two compartments, Fig. 19, p. 17. He then formed a passage way around the diaphragm, which could be opened and closed by mercury, by respectively lowering and raising the 18lower vessel of mercury along the barometer tube. When the passage way is opened around the diaphragm, the luminosity extends through the passage way in preference to going through the diaphragm. When the passage way is closed by mercury, the discharge goes through the thin metal plate. The same was found to occur when the platinum leaf was replaced by a mica scale.
33. Sir David Solomon’s Experiment in 1894. Proc. Royal So., June 21, ’94. Nature, Lon. Sept. 13, ’94, p. 490.—With a tube having a perforated diaphragm, he noticed a “forcing effect” at and near the hole. The striae had the appearance of being pushed through from the longer part of the tube—the diaphragm not being in the centre. There was no passage way around the diaphragm—only through the small puncture. § 19.

34. Riess’s Experiment. Electric Images. Riess’s Reibungs. vol. 2, § 739.—He laid a coin upon a plate of glass and charged the same electrically about one-half of an hour or more. Upon removing the coin and sprinkling the plate with dust, an engraving of the coin was visible upon the glass. § 13. A suitable dust is licopodium powder.
35. Sanford and McKay’s Experiment. Electrographs. Original Contribution by Prof. McKay of Packer Inst., Brooklyn, May, ’96.—The picture of the coins in Fig. IX, was produced by the apparatus shown in Fig. VIII, t, t, tinfoil, p, photographic plate with coins on sensitive side, all wrapped in black paper. Fig. VIII represents the general arrangement for taking electrographs. This particular one was made by removing the upper tinfoil and touching each coin successively with wire from one of the poles, while the other wire was connected with tinfoil on the opposite side. The condenser thus formed is charged and discharged many times by a Holtz machine or induction coil. This is not a new discovery, it was first described by Prof. Sanford, I think, of Leland Stanford University, two or three years ago. Other claimants of earlier date probably exist.
36. Lichtenberg’s Experiment. Dust Figures. Pictures Drawn with Anode and Cathode. Göttingen, 1778-79. Motum Fluidi Electriciti.—He drew two independent superposed pictures upon a flat surface of an insulating material, for example, rosin. One picture was drawn with one terminal of a charged Leyden jar. Another picture was drawn with the other terminal of a charged Leyden jar. He sprinkled upon the surface over the two pictures, a dust made of a mixture of red lead and sulphur powder. The former became attracted to the picture drawn with the cathode, and the latter to that made with the anode, so that the two figures were clearly visible. Before sprinkling the powders upon the surface it is necessary to stir them together whereby they become oppositely electrified.

X
The sulphur arranges itself in tufts with diverging branches and the red lead in small circular patches. The particular materials, namely, the sulphur and red lead were first used by Villarsy. In case only one powder is employed, for example, licopodium, it adheres to both the positively and negatively electrified portion of the insulating plate, but in larger quantities upon the latter portions. Fig. X, shows rosin disc covered with licopodium powder after touching the disc with the knob of a Leyden jar.
36a. Hammer’s Photo-Electric Dust Figures. From personal interview.—According to experiments of Elster and Geitel, hereinafter noted, § 98, Hammer’s dust figures shown in the accompanying half-tone cut may possibly be accounted for on the principle of the discharge of negatively electrified bodies by light. Mr. William J. Hammer, Mem. Amer. Inst. Elect. Eng., has a historical collection of incandescent lamps (Elect. Eng., N.Y., April 29, ’96, p. 446.) which were arranged on shelves in a glass case standing obliquely in the sunlight about an hour a day. After the lapse of many months, the very fine dust within the case lodged upon the inner surface of the glass in such a manner as to produce oval dust figures corresponding somewhat to the shapes of the lamps and some of them, appear after reproduction by the half-tone process in the accompanying cut. When the figures are inspected closely and the circumstances are known, no one can doubt that the sun and lamps acted as agents in their formation. As to the correct explanation, the matter has not been sufficiently discussed by scientists (presented here for the first time) to enable the author to render the opinions of others, but it is of interest in connection with Roentgen rays and the discharge of electrified bodies by light. As a matter of course, the surfaces of the lamps would reflect the light in such a way as to make bright spots (movable, however, with the sun) upon the glass of the containing case, and if the latter were in any sense charged by negative atmospheric electricity, this light would cause a variable amount of dust to be attracted according to the intensity of the rays striking the glass. These remarks are in the nature merely of a suggestion of a hypothesis. The heavy curved black line in the cut is a part of the frame of the glass case. The incandescent lamps do not show, simply because the case was empty when the photograph was taken. 22That the figures were not due to chemical action was shown by rubbing off some of the dust with the fingers. Finger marks were pictured on the figures. Off hand, Mr. Hammer and Prof. Anthony intimate air convection by differentiation of temperature, as a possible cause.

2336b. Independently of the above peculiar phenomenon, Mr. Hammer recently had on exhibition at the Electrical Exposition of the National Electric Light Association in New York, 1896, a portrait formed of fine dust upon a pane of glass. The circumstances were as follows, as remembered by the author. Mr. Hammer happened to be in some place where an artisan was removing a photograph from an old frame. The glass which protected the portrait exhibited a fac-simile in dust on the inner surface. The glass had not been in contact with the photograph, because of a thick passe-partout surrounding the picture. Neither was the glass an old negative photographic plate. Further test and inspection tended to prove that the dust picture was executed by some action of the heat or light of the sun. Prof. Benjamin F. Thomas, of the University of the State of Ohio, in an interview, scarcely thought that the result was due to convection, because the dust print was so sharply defined. The principle of the discharge of bodies by light may be applicable perhaps, but further experiment would be necessary as a more secure foundation. It is common to find the print of a picture in a book upon the opposite page, being due merely to the pressure of the inked surface, as in the art of printing. This explanation cannot be applied to the dust portrait, because there was no contact between the photograph and the glass.
37. Karsten’s Experiment. Electrical Images Developed by Condensed Moisture. Riess’s Reibungselect., vol. II., § 739.—He arranged the following articles in the following order: First, a metal plate suitably insulated; secondly, a piece of a glass plate on top of the metal plate, and, thirdly, a coin or small metal object on top of the glass. Sparks were then allowed to pass for several minutes from a Holtz or similar machine to the coin. The image of the latter appeared by removing the glass plate and breathing upon it. The bas-relief of the image on the coin also was visible in all its details, appearing as in Sanford’s Electrograph, § 35. Theoretical considerations led others to believe that the figures of Riess and Karsten are due to a different cause from that involved in the figures of Lichtenberg, for the former are thought to be due to a molecular action of a permanent nature upon an insulating material. A slight change in the color often occurs, thereby outlining the object.

Dust-portrait on Glass, § 36., p. 23, discovered by William J. Hammer.
Lighter portions, dust; darker portions, due to less or no dust. Finger-marks across the shoulder and at right. Exposure 8 years. Portrait as sharp and clear as a daguerreotype. During exposure in frame, distance of glass from photograph, 1/16 inch. Above half tone was made from a photograph of the dust-portrait only after several unsuccessful attempts by different photographers. The original dust-portrait is scarcely visible. Let every one examine closely glass plates when taken from old frames.
2537a. McKay’s Experiment. Magnetographs. From Personal Notes by Request. April, 1896.—Although this experiment does not belong to that class connected with discharge tubes, yet the phenomenon has a theoretical interest in connection with X-rays. He obtained a photograph of different objects in the dark by means of radiations from the poles of an electro-magnet after two hours’ exposure, but it need not have been so long, as he obtained clear images in five minutes in one experiment with frequent variations of current by means of a rheostat, and by approach and recession of the armature. The elements involved in the experiment were arranged in the following order: First, a large inverted magnet for supporting 100 lbs., the poles hanging downward. Next in order was a wooden board pressing flatwise against the ends of the poles of the magnet. Next, the objects and the sensitive plates backed thereby and all enclosed in a completely opaque wrapping extending over the sides, face, back, etc., of these two elements. Next in order was an armature about as heavy as the magnet would support. The cut herein represents the photograph that was produced of the different objects named. By reading Prof. McKay’s very detailed description in the Scientific American, April 18, 1896, p. 249, the reader may feel certain that the photograph was not due to light for he tried the experiments in different ways and with various precautions. In a course of experiments carried on by student Austin, about Feb. 15, ’96, in the Dartmouth laboratory, a sciagraph of what appeared to be the lines of force was obtained by means of X-rays, but upon repeating the experiment the result was negative. See Elect. Engineer, Mar. 11, ’96, p. 257. Article by E. B. Frost.
XI
38. Piltchikoff’s Experiment. Liquid Bas-relief Facsimiles by Electric Discharge. Pro. Acad. Sci., Paris, March, ’94. The Electr., Lon., April 13, ’94, p. 656.—These shadow pictures were obtainable either with the anode or cathode, the particular machine employed being a large Voss. To either pole was electrically connected a pointed wire which was held just above the surface of castor oil, in a copper pan. A remarkable effect was obtained of the shadow of a piece of mica, Fig. XI, of whatever shape, located between the point and the surface. § 24. Let it be observed that this shadow was not one in the sense of light and darkness but it consisted of a plateau within a depression, the former being of the same 26shape as though it were a shadow of the mica triangle. To illustrate the experiment better, let the mica be supposed to be removed, then will there be a depression formed in the oil upon bringing the metallic point near to the surface. Now insert the insulating sheet between the point and the surface, then will there be an elevation within the depression of the same shape that the shadow would be.
39. Gernez’s Experiment. Distillation of Liquids by Discharge. Phys. So., Paris, 1879. Nature, Nov. 20, 1879, p. 72.—In order that the apparatus with which he experimented may be understood, imagine a tube standing vertically in another tube. The two concentric tubes communicate with each other at the top only. The Holtz machine is the generator. The liquids in the two tubes at the beginning stand at the same level. Sparks are passed through the adjacent air, which is in contact with both liquids. The liquid at the cathode rises and at the anode falls. § 38. Such was the experiment performed by Gernez. He was inclined to conclude that the effect was due to “An electrical transport of liquids along the moistened surfaces of the tubes.” When the liquid was alcohol, it actually went over as by distillation, three times as fast as water. A soluble salt in water increased the rate of distillation; and so also did the addition of a small quantity of sulphuric acid or ammonia. No distillation of bi-sulphide of carbon, tetra chloride of carbon, nor turpentine occurred. Query: Can alcohol be concentrated or practically distilled upon this principle?
40. De La Rue and Müller’s Experiment. Striae. Black Prints on Walls of Tube. Phil. Trans., 59, ’78.—Particles of the metal of the electrodes were deposited upon the inside of the glass forming permanent black striae or bands § 44, at points corresponding to the spaces between the luminous striae. § 6. near the end.
41. Gassiot’s Experiment. Striae. Tube in Primary Current. Current Vibratory. Phil. Trans., ’59, p. 137. Bakerian Lectures. Phil. Trans., ’58, p. 1. Proc. R. So., x., pp. 36, 393, 404; xii., p. 329; xxiii., p. 356.—The form of tube in which to obtain luminous striae to the best advantage was that of a dumbbell with the electrodes located respectively in the balls—afterwards confirmed by Sir David Solomons, Bart. Proc. Royal So., June 21, ’94. Nature, Lon., Sept. 13, ’94, p. 490. He obtained in the vacuum luminosity with 500 Daniell’s cells, which he found to be the least E. M. F. that could be employed. He omitted, and apparently overlooked, the introduction of an automatic interrupter in the circuit and the use of a very low E. M. F. § 52. In conjunction with Spottiswoode, 1,080 cells of chloride of silver (about 2,000 volts) were employed, first without, and then with condensers. One of the condensers consisted of the usual tinfoil type, and the other of a self-induction kind, namely of about 1,000 feet of wire. The results were striae with the condensers, and no striae without the condensers. § 8a. The results suggested to them that there was some relation in principle between the striae and vibration of the current. They therefore built an ingenious apparatus to test whether this was true or not, and they found such was the case by the following related means. If a current passing directly from the primary battery through the condenser and the discharge tube is undulatory or intermittent in any sense, then it would be able to induce a current in the secondary of the induction coil. § 8 at centre. They found that there was a current thus induced, and they detected it by means of a small discharge tube which became luminous. Fig. 3 p. 17. This was an independent tube near the top of the figure, having nothing to do with the one containing striae, which were produced by the primary current and shown at the right. Dr. Oliver Lodge, F.R.S., in treating of the cathode and X-rays in The Elect., Lon., Jan. 31, ’96, p. 438, stated the following with reference to Gassiot’s experiments: 28“In the days of Gassiot and other early workers (§ 43) on the discharge in rarefied air, it was the stream from the anode that chiefly excited attention. It is this which developed the well-known gorgeous effects which used to be shown at nearly every scientific conversazione.”
42. Poggendorff’s Experiment. Effects of Interrupting a Current Within Discharge Tube. Phil. Mag., 4th Se., vol. x., 1855, p. 203-207.—Imagine an electric bell vibrator and magnet within the glass receiver upon an air pump. Upon connecting the magnet and vibrator in series with a small electric battery, it is evident that in the open air, as usual in electric bells, there will be a minute violet spark at the terminals of the circuit breaker. § 6. Now, let the air be exhausted as far as possible by means of a mechanical pump as constructed in 1855. Poggendorff performed such an experiment, and he noticed that in the poor vacuum the ordinary violet spark became yellow, while blue light like a small enveloping tube surrounded the hammer of the vibrator and wire leading to the opposite contact and a little projection extending away from the hammer. His experiment was unique, because showing for the first time that a current from a battery, if interrupted in the vacuum, will not only produce the usual minute spark, but that a blue tube of light will be produced around the conductors within the vacuum.
43. De La Rue and Müller’s Experiment. Source of the Striae at the Anode. Number of Striae Varied by Change of Current. Phil. Trans., 1878.—By an arrangement of means for causing different pressures, they made a discovery, namely, that as far as the eye is concerned the striae begin to have their existence at the anode. § 46. Imagine the internal gas pressure to become less and less. First, a violet luminosity occurs around the anode as in § 42. As the pressure becomes less and less, luminous striae move toward the cathode accompanied by more and more striae, which increase either to form a column reaching a certain distance or else extending through the whole distance between the electrodes. § 46. They observed that when the E. M. F. was constant and the current changed, the variation in the appearance of the striae was very regular. § 41. With some tubes the number of striae increased with the increase of current, while with a decrease of current the number of striae became less and less. § 8a. With some tubes the number of striae increased while the current decreased. § 8a. With the use of a condenser, then as the E. M. F. decreased together with a diminution of current, the number of striae varied. The 29striae nearest the anode vanished first, as they diminished in number with the fall of the E. M. F. The striae on the other hand originated at the anode, when the charge of the condenser was gradually increased from a minimum, and then the striae continued to increase from the anode as the source. As to the color of the striae, the same was changed by an alteration of the current.
44. Solomons’ Experiment. Dark Bands by Small Discharges. Nature, Lon., Sept. 13, ’94. Proc. R. So., June 21, ’94.—Solomons found that in a very dark room, striae (alternate light and darkness) appeared with very minute discharges, and as the current was increased, they vanished, appearing again when the discharge was strong. He could not obtain them until the luminous column extended to the glass forming the large glass tube. § 40.
45. Spottiswoode’s Experiment. Governing the Motion of Striae. Effect Upon Motion by Diameter of Discharge Tube. Motion Stopped by Magnet. Proc. R. So., vol. 33, p. 455.—Spottiswoode found that he could obtain motion when he desired. He introduced some constant resistances and also a rheostat of fine adjustment. The least change of resistance caused some effect upon the striae. The general principle that he established was that letting it be assumed that the striae are stationary then; “An increase of resistance produces a forward flow, and a decrease of the resistance a backward flow,” differences of as little as 1 ohm in the primary current caused the effect. Sometimes the velocity of the flow is fast and sometimes slow, being so rapid in certain instances that the unaided eye cannot distinguish them, but they are known to exist by the use of the revolving mirror. § 46. With tubes of small diameter, compared with their length, he noticed the fact that the striae in one portion of the tube moved faster than those in another portion. § 46. Sometimes one group moved while the other one was stationary. Sometimes they moved in opposite directions. This last named phenomenon occurred also in very wide tubes. The points at which the charge took place he called nodes. He discovered external means for stopping this action. He did it by means of a magnet located opposite one end of the tube. § 31. When the magnet was energized, all motion ceased. § 31.
46. Thomson’s Experiment. Velocity of Striae Checked at the Cathode. Nature, Lon. Jan. 31, ’96, p. 330.—A tube 50 ft. long was exhausted, § 8a., as to striking distance. In this particular experiment, he caused a single interruption in the primary of the induction coil, and observed the motion of the 31striae from the anode to the cathode by means of a rotating mirror. § 43. The luminosity began at the anode and travelled toward the negative with a high velocity, but upon its arrival at the negative pole its velocity was checked. He said that the striae did not disappear at the cathode like a rabbit would in entering a hole, but they lingered around the electrode for some time. As a consequence of this delay, he found as expected, an accumulation of positive electricity, § 4, in the neighborhood of the cathode. It is a general principle, therefore, that when a discharge passes between a gas and metal, there is an accumulation, illustrating that the discharge experiences a difficulty or resistance. § 32 and 33. The experimenter, Prof. J. J. Thomson, acknowledged that Profs. Liveing and Davy had noticed similar effects.
47. Thomson’s Experiment. Disruptive Discharge and Electrolysis. Nature, Lon. Jan. 31, ’95. Lect. S. Inst. The Electr., Lon. vol. 31, p. 291, 316, and vol. 35, p. 578. Trans. R. So., ’95.—The discharge of electricity through conducting liquids is, with scarcely an exception, (example, mercury) accompanied by a chemical action. Faraday and Davy both performed early experiments in this direction. Prof. J. J. Thomson has set forth some instructive facts and which act as evidence that there is a close relation between the disruptive discharge and chemical action between the dielectric and electrodes. § 6 and 7. He made this experiment in connection with his investigations relating to the difficulty the positive electricity experiences in passing from a gas to the negative electrode. § 46. He carried this experiment further, by testing gases of different chemical natures. The apparatus he employed consisted first of an alternating current generator, a high tension converter, a bulb for containing first one gas and then another, whose metal electrodes were connected with the secondary of the transformer, and an electrometer connected to a third electrode which could be moved about within the bulb. The operation was as follows: when the bulb contained oxygen which is an electro-negative gas, the third movable electrode received a positive charge in whatever part of the bulb it was moved to, but with hydrogen instead of oxygen at atmospheric pressure, the third electrode received a positive charge far away from the arc between the other electrodes, but very near the arc it received a negative charge. He then rarefied the atmosphere of hydrogen and he noticed that the space where the third electrode became negative, contracted, and at about 1/3 of an atmosphere became practically nothing, so that the said third electrode connected to the electrometer 32became slightly positive at all points within the hydrogen. § 4. The next step consisted in using a bulb, having oxydized copper electrodes and a hydrogen atmosphere at the pressure where there was only positive electricity, that is about 1/3 of an atmosphere. This remarkable phenomenon occurred; there was no positive electricity, but only negative. When the copper oxide was reduced, the positive electricity only, existed in all parts of the bulb. In brief, bright copper electrodes left a positive charge in the gas, while oxydized electrodes left a negative charge. He argued upon the results of this experiment to account for the delay in the passage of the electricity from the gas to the metal, § 46. In later experiments, he used the spectroscope to detect decomposition. § 6, at end.
48. De La Rue and Müller’s Experiment. Heat Striae. Phil. Trans., vol. 159, 1878—They arranged for the best conditions, that is, when a small number of striae occurred in conjunction with a wide, dark interval. § 44. They found that the heat was greatest at the position of maximum luminosity, but they also found that heat was generated at the dark spaces. A novel feature was the discovery of the development of heat in the middle of the tube even when there was no luminosity, § 9a, near end, so that they thought it probable there may be what might be termed heat striae, independently of luminous striae.
49. Spottiswoode and Moulton’s Experiment. Sensitive State. Air-Gap in Circuit Forms Best Method of Obtaining. Branch Current to Earth Verified by a Telephone. Sensitive State by a Single Quick Discharge. Phil. Trans., 1879, p. 165, and April 8, 1880.—By sensitive state of luminous effects in a Geissler tube is meant the susceptibility of the light (§ 28) to an outside conductor connected to earth. Fig. 5, p. 17. When one’s hand is brought near a Geissler tube the change near the hand sometimes occurs and sometimes it does not. § 8. In the first place, the effect is more easily noticeable if the vacuum tube is comparatively wide or thick in diameter. With the electric egg, for example, the luminous effect, instead of extending more or less across the space between the electrodes, reaches from one of the poles to a conductor on the outside of the egg, provided said conductor has an earth connection or large capacity. Some of the light continues to exist nevertheless between the two poles. The general principle is that the division exists because of the re-distribution or branching of the disruptive discharge. It was not known why the luminosity should be affected by such an outside conductor sometimes, and remain the same at other times but the above named experimenters discovered causes 33which could be depended upon to produce the sensitive state. The apparatus will be described. They had the usual Geissler tube with the platinum wire electrodes, and a Holtz machine as the generator. They were led to believe that intermissions of the current had a great deal to do with the production of the sensitive state, and accordingly they arranged for an air-gap in circuit with the machine and with the vacuum tube. § 51. They not only observed that such a gap caused the sensitive state, but that an increase in the length of the gap made the luminous column more sensitive. They increased the gap so much that the ramifications of the light could be seen. If an induction coil is employed as the secondary generator, a condenser should be coupled up in connection with it. The two in combination thereby produce the sensitive state, but upon cutting out the coil and charging the tubes from the condenser the sensitiveness can not be detected. Instead of the permanent air-gap, may be employed a rapid circuit interrupter, coupled up between a Holtz machine and a vacuum tube. The manner of coupling up is to place the interrupter in a shunt to the vacuum tube. Difficulty had been found in early experiments to obtain the sensitive state with those vacua which give striae. With a rapid circuit interrupter and an induction coil, the breaks occurring 240 per second, the luminous column was not only broken up into striae, but were acted upon by the approach of an outside conductor connected to earth. The sensitive state is not always made apparent by the appearance of attraction of the luminous light to the outside conductor. Sometimes the light seems to be repelled. These two phenomena may be caused in the same tube. This feature of the sensitive state constitutes the beginning of radiations of energy through the walls of a vacuum bulb, like X-rays. Some action or other in these cases takes place through the glass. They tried an experiment in which one of the electrodes of the vacuum tube was entirely on the outside. The electrical discharge was found to be sensitive, for the discharge was changed in its appearances by the presence of an outside conductor connected to earth. Another cause of the sensitive state was observed, namely, the brevity of the charge. This may be illustrated with a Leyden jar, which is known to give an almost practically instantaneous discharge. A single discharge from such a jar produced a flash of light which was in the sensitive state. The nomenclature by which the experimenters defined the cause of the phenomena is made up of the words: Re-distribution of electricity, and a relief of the external strain.
3449a. No re-distribution took place unless the outside conductor was connected to earth or to a conductor of large capacity, nor would an outside conductor, which was already charged, serve to exhibit the sensitive state. The re-distribution effect was proved by means of a telephone connected in circuit between the outside conductor and the earth Fig. 5, p. 17. When the state was sensitive, that is, during the use of the air-gap, the telephone produced a sound in unison with the intermissions occurring at the air-gap. § 9 and 9a.
50. Reitlinger and Urbanitzky’s Experiment. Sensitive State Illustrated by a Flexible Conductor Within the Discharge Tube. Proc. Vienna Acad., 1879. Nature, Nov. 20, 1879.—The discharge tube was 20 cm. long. It had the usual platinum electrodes, and it stood upright. From the upper electrode, was suspended a strip of tinfoil in the middle of the tube, which was connected to a pump so that the density of the gas could be varied. At atmospheric pressure, the secondary current of a Ruhmkorff coil connected to the electrodes caused the strip to be attracted to the glass tube. The attraction was less and less as the process of exhaustion was carried on, and when a pressure indicated by 7 mm. was reached, the strip was neither attracted nor repelled, but hung downward the same as without any electricity whatever, but it was attracted by a neighboring shell-lac rod which had been rubbed with cloth, and it was repelled by a glass rod which had been rubbed with amalgam, it being assumed that the strip was connected to the anode. § 36. The opposite action took place when it was connected to the cathode. As the exhaustion continued and became greater and greater, these actions died away also up to a rarefaction of about 4 mm. Independently of the degree of rarefaction, the flexible strip of tinfoil was always deflected by an outside conductor connected to earth. § 49.
51. Tesla’s Experiment. Incandescent Electrode by High Potential and Enormous Frequency. System Referred to by Roentgen for Generating Powerful X-Rays. U. S. Letters Pat., No. 454, 622, June 23, ’91. Martin’s Researches of Tesla; Trans. Amer. Inst. Elec. Engineers, May 20, ’91; Elec. Review, N.Y., June 24, ’93, p. 226; Lect. Franklin Inst., Feb. 24, ’93, and Nat. Elec. Light Asso., Mar. 1, ’93; also Lect. in Europe. Later he again experimented in this direction, see Elec. Review, N.Y., May 20, ’96, p. 263.—By the U. S. Patent Office he was granted, among other claims, the following: “The improvement in the art of electric lighting herein described, which consists in generating and producing for the operation of lighting devices, 35currents of enormous frequency and excessively high potential, substantially as herein described.” A simple combination of circuits together with great skill in the construction of apparatus involving high powers of insulation, resulted in the production, within a vacuum, of an electrode radiating intensely white light. The circuit may be easily traced in the diagram Fig. 17 p. 17. Briefly described, there may be noticed an alternating current generator of comparatively low E. M. F. The current from this generates a secondary current by means of an induction coil. This secondary current generates a tertiary current by a second induction coil. An air-gap for automatic and intermittent disruptive discharges, § 49 near end, is in the circuit of the secondary coil of the first named induction coil, which is directly charged by the alternating current generator. The gap may be noticed between the two balls. In shunt to the air-gap is a condenser (see Fizeau, chapter I.) represented by several parallel lines. The lamp consists merely of an evacuated bulb having an electrode of carbon or other refractory material, which is connected to one pole of the last secondary coil while the other pole may be outside, and may consist, for example, of the walls of a room, which in such a case should be of some electric conducting material. The higher the vacuum the more intense the light; he found no limit to this rule. Fig. 16a p. 17 illustrates his ideal method of lighting a room. He found that with two plates at a distance apart as indicated and connected to the poles of the coil, and with electrodeless vacuum bulbs, the latter became bright in space—no mechanical or electrical connection other than air and the assumed ether.
52. Moore’s Experiment. Luminosity in Discharge Tube by Self-induced Currents. Trans. Amer. Inst. Elect. Eng., Sept. 20, ’93 and April 22, ’96. Several U. S. Letters Patent. Invented 1892.—During or about 1831, Prof. Henry discovered that when the circuit of a primary battery was interrupted, a self-induced current, which he called an extra current, was produced. When the circuit was closed, there was also a self-induced current, but very much feebler than that obtained on interruption. The self-induced current occurred only at or about the instant of interruption or completion. He found also that the self-induced current produced by interruption was enormously increased in E. M. F. if the circuit included a helix of very long and fine wire. It was further increased by the presence of an iron core. With one or two cells, the spark upon interruption was scarcely visible, but with a fine wire 30 or 40 feet long, an appreciable spark was obtained during interruption. With but a comparatively 36few cells, and with a magnet for example like a telegraph relay, the E. M. F. arose to several thousand volts at the instant of interruption. D. McFarland Moore introduced into such a circuit a Geissler tube and provided a rapid automatic interrupter. Page, Ruhmkorff and others had, at an early date, noticed the desirability, in operating Geissler tubes by secondary currents, to obtain quick interruption in the primary circuit in order to produce the best effects in the Geissler tube. Moore caused the interruptions to take place in a vacuum, so high that a disruptive electrical discharge could not pass. The break was therefore, absolutely instantaneous and complete. By this system, illustrated in diagram in Fig. 18, p. 17, he obtained all the luminous effects, actions by magnets, the sensitive state, striae and all the other phenomena heretofore noticed in Geissler tubes and some of those obtained by Tesla with his apparatus as just described. In greater detail, it will be noticed that he had a dynamo of rather low E. M. F., generally 100 volts, and a high vacuum containing a circuit interrupter operated automatically by a magnet outside like a vibrator in an electric bell. The magnet served also as the self inductive device. The magnet and interrupter were in series with each other, therefore, while the Geissler tube was in series with the magnet, and the electrodes extended either inside of the Geissler tube or remained on the outside. He performed numerous experiments on similar lines and developed the system on a large scale, whereby rooms (e.g. the hall of the Amer. So. Mech. Eng., N.Y.) have been illuminated as if by other artificial illuminants, by employing long and numerous vacuum tubes. Among several discoveries was that of the production of a bright pencil of light along the axis of a long open helix, which formed one of the internal electrodes. The Patent Office made strenuous efforts to determine the degree of novelty, assuming that some one else must have conceived the idea of employing a self-induced current to operate Geissler tubes; but nothing nearer than Poggendorff’s experiment § 42 could be found, and therefore the following claim (in patent 548576, Oct. 22, ’95,) was granted among a hundred or so relating to developments and details and particularly covering the vacuum interrupter. “The method of producing luminous effects, consisting in converting a current of low potential into one of high potential, by rapidly and repeatedly interrupting the low potential current in its passage through a self-inductive resistance, and passing the former current through a Geissler tube, thereby producing light within the tube.”

53. Crookes’ Experiment. Dark Space Around the Cathode. Lect. Brit. Asso., Shef., Eng., Aug. 22, ’79.—According to Lenard (The Electr., Lond., Mar. 23, ’94) Hittorf discovered the cathode rays, and Varley, § 61a., and Crookes studied them. The pressure of the residual gas was 1 M. of an atmosphere. Prof. Crookes, F.R.S., maintained the evacuated space in communication with the air pump and with an absorbent material. Before his time most experimenters worked with a vacuum not much less than 30,000 M. The first experiment is illustrated in diagram, at Fig. 6 p. 17, but the vacuum was not the highest in this type. The tube was cylindrical and was provided with electrodes at the ends. Another electrode was located at the centre and was made the cathode, while the two terminal electrodes were made the same pole; namely, the anode. Upon connecting the tube in circuit with the secondary of a large induction coil, the luminosity did not extend either continuously or in striae throughout the length of the tube. Former investigators had likewise noticed the dark space. The space and glass on each side of the central cathode were dark. The dark space extended for about one inch on each side of the negative pole. It is not intended here, any more than in former cases, to present theories in explanation further than to briefly allude to any conclusion at which the experimenter himself arrived. Crookes’ explanation of the phenomena has not been universally accepted, nor has it been proved otherwise. The knowledge of the existence of rays, now known as Roentgen rays, will assist in formulating theories upon the Crookes’ phenomena and may either confirm some of his views or overthrow them. Crookes considered that the residual atmosphere was in such a state as to be as different in its properties from gas, as gas is from liquid and liquid from solid, and therefore he named the attenuated atmosphere radiant matter, or fourth state of matter. He concluded that the remaining particles of the gas forming the radiant matter moved in straight lines over a great distance as 39compared with that moved through by molecules at the ordinary pressure. He called this distance the “mean free path.” If his theory is correct, this dark space is due to the fact that the molecules in motion at and near the cathode do not bombard each other and therefore do not produce the effect of light. When the motion is arrested by particles of gas themselves, within the bulb, then is light generated. The force propelling the particles from the positive pole was supposed to be less. In order to let the experiments speak for themselves, as much as possible, without being too much influenced by the opinion of the experimenter; the theory is only briefly alluded to as above, and will not be further applied in the presentation of his other experiments. In view of the radical discoveries of Lenard and Roentgen, after the installation of the Crookes phenomena, it has been the policy of the author to present all the experiments as facts for evidence in behalf of the general theories, which may be hereafter formulated independently of old theories. Therefore, the reader should bear in mind the teachings of the various experiments with the view of arriving at general principles and hypotheses.
54. Relation of Vacuum to Phosphorescence.—He started with such a high vacuum that he could not obtain any electrical discharge. § 25. There was, therefore, no phosphorescence in the glass tube, whatever. The caustic potash, which had been employed to absorb the last trace of moisture and carbonic acid gas, was slightly heated, and very gradually. Then it was noticed that a current began to pass and that the glass became green, and apparently on the inner surface. As the heat continued, the green passed gradually away and was replaced by striae, which first appeared to extend across the whole diameter of the glass tube (§ 40) which was a long cylindrical tube, and then became concentrated toward the axial line of the tube. Finally, the light consisted of a pencil of purple. § 10. When the source of heat was removed so that the moisture and carbonic acid gas could be absorbed again by the potash, the striae appeared, and then the other effects just named, only in the reversed order, until the tube acted like an infinite resistance. Phosphorescence is the correct word, because the light existed for a few seconds after cutting off the current.
55. Phosphorescence of Objects Within the Vacuum Tube.—The construction in Fig. 7, p. 17, shows how a diamond was caused to phosphoresce within a Crookes’ tube, being supported in a convenient manner in the centre of one of the tubes, while electrodes were located near the ends and were formed of 40disks facing the diamond. Upon connecting the disks to the respective poles of the secondary conductor, and by performing the experiment in a rather dark room, the diamond became brilliantly phosphorescent, radiating light in all directions. He experimented with many substances in this way, but found that the diamond was the best—almost equal to one candle power. In order to exhibit the phosphorescence of glass in a striking manner, he charged three small tubes simultaneously. One was made of uranium glass which radiated a green light. Another was an English glass which appeared blue, and the remaining one was German glass which phosphoresced a bright green. Notice difference with respect to light which does not perceptibly cause phosphorescence of glass. The uranium glass was the most luminous. Luminous paint, as prepared by Becquerel, and later by Balmain, which has the property of storing up light and afterwards radiating it in a dark room for several hours, became more phosphorescent in the Crookes tube than when subject to day-light. Phosphorescence of the mineral phenakite, the chemical name of which is glucinic aluminate, was blue, the emerald, crimson, and spodumene, which is a double silicate, were yellow. The ruby phosphoresced red, whatever its tint by day-light. In one tube he had rubies of all the usual tints by day-light, but they were all of one shade of red by the action of the disruptive discharge in the tube.
56. Darkness and Luminosity in Arms of V Tube. See Fig. 8, p. 17. It will be noticed that in Fig. 6, p. 17, the tube was straight. Crookes desired to see what effect would take place in a bent tube. He therefore employed a V shaped tube, having electrodes in the ends—one in each arm. Upon causing the electrical discharge to take place through the tube, one arm was luminous and the other was dark. Whatever the E. M. F. was, the appearances remained the same. No luminosity would bend from one arm of the V shaped tube to the other. The cathode arm was always luminous and the anode dark. With a less degree of vacuum, both arms were luminous, according to early experimenters who thus brilliantly lighted tubes of the most fantastic shapes.
57. Cathode Rays Rectilinear. Radiate Normally From the Surface of the Cathode. In his lecture he had, side by side, two bulbs, one, in which the vacuum was of such a degree, that a blue stream of light existed between the negative pole and positive pole, § 54, at centre. It is evident that the vacuum in this bulb was not very high. Fig. 9, p. 17, shows a stream extending from the negative to the positive pole, Fig. 10, p. 17, 41is the same kind of a tube only the vacuum is about 1 to 2 m. In other words, the vacuum in the latter was just so high that a discharge took place, and instead of the luminous effect being like that with a low vacuum, there was a patch of green light directly opposite the concave negative pole. The radiations from this pole were rectilinear, crossing each other at a focus within the bulb and producing upon the glass a phosphorescent spot. It should be remembered that the word radiations is used as a mere matter of convenience. Directly opposite the concave cathode, there was a green patch of light on the inner surface of the glass. It was shown that it made no difference where the anode was. This fact becomes useful in carrying on experiments in connection with Roentgen rays, and it may have a great deal to do with the solution of the theoretical problems in connection with electrical discharges in vacuum tubes. In regard to the three streams shown in Fig. 9, p. 17, it may be stated that only one occurred at a time in the experiment, for, first one anode was connected in circuit, and then the next by itself, and then the third one by itself, while the concave pole was always negative. Each time the anode was changed, the stream changed, and connected that pole which was in circuit, § 43, but similar changes made upon the tube with a high vacuum, did not alter the position of the phosphorescent spot. This and other experiments show that the radiations took place perpendicularly from the surface of the cathode.
58. Shadow Cast Within the Discharge Tube. This is illustrated in Fig. 11, p. 17, where there is a negative polar disk at the small end of the egg shaped tube, and a cross near the large end, the same forming the positive pole. The cross is made of aluminum. There was a novel action, however, discovered in addition to the mere casting of a shadow. The glass which had become phosphorescent except within the shadow, became after a while, less phosphorescent. Its property to phosphoresce became less as proved by removing the cross, which was arranged to fall down upon tipping the bulb. Immediately, the part which was within the shadow became brighter than the rest of the glass, thereby reversing the appearances, by making a luminous picture of the cross upon only partially phosphorescent glass. A remarkable feature is that the glass never recovered its first exhibited power of phosphorescence, neither did this power entirely become nothing, however many times the tube was employed. Was the deposit of metal from the cathode the cause?
4258a. Mechanical Motion Produced by Radiations from the Negative Pole. It occured to Crookes that the radiations from the cathode might perhaps cause a wheel to turn around. He therefore had a minute wheel made by Mr. Gimingham, like an undershot water wheel, and its axle rested on two rails of glass, so that it might roll along from one end of the tube to the other. The vanes were exactly opposite to the plane surface of the cathode. The molecular stream or radiations, or whatever they may be, possibly vibrations, from the cathode, were so powerful mechanically that the wheel was caused to run up hill, the tube being inclined very slightly. On the principle that action and reaction are equal, he built another device in which the negative electrode was movable, and he observed that when the current was on, the negative electrode moved slightly. Upon these principles he built the well-known Crookes radiometer in which the vanes rotated by reaction of the radiations. The vanes in this form of radiometer were made of aluminum, and a cup of hard steel served as the bearing, Fig. 12, p. 17. One side of each disk was coated with a thin scale of mica. The aluminum disks formed the cathode, while the anode was located at the top. The operation consisted in connecting the terminals as stated, so that the vanes were the negative poles and it was observed that the little wheel rotated. The vacuum was not as high as that for obtaining phosphorescence. With a low vacuum, an envelope of violet light existed near the surface of the aluminum vanes. Effects were carefully studied by maintaining connection with the pump. At the pressure of .5 mm. there was a dark cylinder opposite the aluminum extending to the glass, and this was the pressure at which the vanes began to rotate. The dark spaces opposite each vane became larger and larger in width, until they appeared to be opposed or resisted by the inner surface of the glass, and then the rotation became very rapid. He modified this experiment by having vanes entirely of mica, and by having the cathode disconnected electrically from the vanes, Fig. 13, p. 17. A coil of metal near the vanes served as the cathode. The anode was at a distance in the top of the tube as in Fig. 12, p. 17. During the electrical discharge, the wheels rotated by radiations from the coil which formed the cathode. He made the discovery that when this coil was heated red hot conveniently by a current from a primary battery, the vanes also rotated, showing that there is probably some relation between the radiations from the cathode and heat rays. The fact remains however, that both kinds of rays produced rotation, directly or indirectly.
4359. Action of Magnet Upon Cathode Rays.—He had two tubes, one of which is shown in Fig. 14 and the other in Fig. 15, on page 17. In the former, the vacuum was so low that a violet stream of light existed between the electrodes. In the other, the rays were invisible, but were converted into luminosity by projection at an exceedingly slight angle, upon a phosphorescent screen arranged along the length of the tube and inside thereof. Inasmuch as the whole surface of the cathode in the latter case radiated parallel and invisible rays, he cut off some of them by a mica screen having a hole in the centre and located near the negative pole, so that only a pencil of invisible rays could go through the mica screen and act upon the phosphorescent screen. In both cases, there was visible a straight pencil of light. Now notice the effect which took place upon locating a magnet as indicated in the figures. With the low vacuum, the pencil was bent out of its course but returned again to the line of its original path. § 28. With the high vacuum, the rays were bent but did not return to their original direction nor parallel thereto. In the former case, the magnet acted as upon a very delicate flexible conductor, while in the latter, it acted, as Crookes said, like the earth upon projectiles. He modified the latter experiment in order to determine if the similarity between this phenomenon and gravitation existed in other respects. He anticipated that if the molecular resistance to the rays were increased they would be bent more out of their course like a horizontally projected bullet. He therefore heated the caustic potash sticks slightly, and in view of the liberation of molecules of water within the vacuum tube, the rays, he thought, would be resisted; and such was the case to all appearances, for then the pencil of light was bent out of its course to a greater extent, although the magnetic power remained the same as well as the E. M. F. producing the electric discharge. He therefore established, apparently, the principle that the magnetic actions upon cathode rays vary somewhat in their nature according to the degree of vacuum. In either case, it may be stated incidentally, that when the magnet was moved to and fro, the pencils of light waved back and forth.
In the modified form of construction over that shown in Fig. 15, p. 17, he caused a wheel to rotate that was located in the high vacuum. The vanes of the wheel were so located that the faces of the same were perpendicular to the direction of the pencil of the rays radiating from the cathode. When the magnet deflected the rays, the wheel ceased rotation.
60. Mutual Repulsion of Cathode Rays.—If the little mica 44screen, as shown in Fig. 16, p. 17 has two holes, and if there are two cathodes instead of one, there will also be two pencils of light. He performed an experiment involving the latter modification, and the result was something that could not have been predicted. The two pencils, as displayed by the long fluorescent screen, repelled each other like molecules similarly electrified. The white pencils, it will be noticed, were repelled from each other and showed their condition when both of the negative poles were in circuit. The black pencils show the location of both of the pencils when only one pole is in circuit at a time, the direction being perpendicular to the plane of the cathode disc (§ 57) at end.
61. Heating and Lighting Power of Cathode Rays. Heat of Phosphorescent Spot.—By making the cathode concave as in Fig. 10, p. 17, and so locating it that the focus of the cathode rays falls upon some substance, the latter becomes very hot. In this way Crookes melted wax on the outside of the bulb at the phosphorescent spot. Further than this, the heat was so great that it cracked the glass without at first injuring the vacuum; next the glass at this point softened, and the air, by its pressure, rushed into the bulb, forcing a hole through the soft part. He performed an experiment also which illustrated the intensity of the heat when the rays were brought to a focus. He used an unusually large electrode like a concave mirror, and in the focus, which was near the centre of the bulb, he supported a small piece of iridio-platinum. At first, with a moderately low E. M. F., the metal was made white hot. When a magnet was caused to approach, the rays were drawn to one side, § 59, and the little piece of metal cooled. He then put in all the coils of an inductorium, and allowed the metal not only to become white hot, but to become so heated that it melted. How little did Prof. Crookes know about the most important phenomena associated with his experiment. Although he was so exceedingly enthusiastic and ingenious in planning his experiments, and in reasoning, yet it seems almost mysterious that he should have been subjected to what have become known as X-rays, which passed into his body, and would have photographed portions of his skeleton, and which would have performed outside of the tube many of the acts that were noticed within. Seventeen years elapsed between the time of Crookes on the one hand, and Lenard and Roentgen’s discoveries on the other. Dr. Lodge, F.R.S., (The Elect., Lon., Jan. 31, ’96, p. 438,) and Lenard, in his first paper, attributed to Hittorf the discovery of the mere existence of cathode rays, but credited to Crookes the full establishment of their properties, 45deduction of their principles and formulation of an ingenious theory.
61a As an appropriate conclusion to Crookes’ work, I cannot do better than to let Lord Kelvin repeat what he said in his Pres. Addr., Ro. So., Nov. ’93, see also The Elect., Lon. Feb. 14, ’96, p. 522, showing that a small portion of the credit is due not only to Hittorf, § 53, but to Varley. “His short paper of 1871, which, strange to say has lain almost or quite unperceived in the Proceedings during the 22 years since its publication, contains an important first instalment of discovery in a new field, the molecular torrent § 53, at centre, from the ‘negative pole,’ the control of its course by a magnet, § 59, its pressure against either end of a pivoted vane of mica, § 59, at end, and the shadow produced by its interception by a mica screen, § 58. Quite independently of Varley, and not knowing what he had done, Crookes (Roy. Inst. Proc., April 4, ’79, vol. LX, p. 138. Ro. So. Trans., ’74, “On attractions and repulsions resulting from radiation” Part II, ’76, parts III and IV, ’76, part V, ’78, part VI, ’79) was led to the same primary discovery, not by accident and not merely by experimental skill and acuteness of observation.” * * * * “He brought all his work more and more into touch with the kinetic theory of gases; so much so, that when he discovered the molecular torrent he immediately gave it its true explanation—molecules of residual air, or gas or vapor projected at great velocities (probably, I believe not greater in any case than 2 or 3 kilometers per second, § 61b), by electric repulsion from the negative electrode. This explanation has been repeatedly and strenuously attacked by many other able investigators, but Crookes has defended (Presidential address to the Inst. Elect. Eng., 1891.) it, and thoroughly established it by what I believe is irrefragable evidence of experiment. Skillful investigations perseveringly continued brought out more and more wonderful and valuable results; the non-importance of the position of the positive electrode, § 57, near end, the projection of the torrent perpendicularly from the surface of the negative electrode, § 57, at end; its convergence into a focus and divergence thenceforward when the surface is slightly concave, § 47, near beginning; the slight but perceptible repulsion, § 60, between two parallel torrents due, according to Crookes, to negative electrifications of their constituent molecules; the change of the direction of the molecular torrent by a neighboring magnet, § 59. the tremendous heating effect of the torrent from a concave electrode when glass, metal or any ponderable substance is placed in the focus, § 61. the phosphorescence procured on a plate coated with sensitive 46paint by a molecular torrent skirting along it, Fig. 15, p. 17; the brilliant colors—turquoise blue, emerald, orange, ruby-red—with which grey, colorless objects, and clear, colorless crystals glow on their struck faces when lying separately or piled up in a heap in the course of a molecular torrent, § 55. “electrical evaporation” of negatively electrified liquids and solids, § 59. (Ro. So. Proc., June 11, ’91.) the seemingly red-hot glow, but with no heat conducted inwards from the surface, of cool solid silver kept negatively electrified in a vacuum 1/1,000,000 of an atmosphere, and thereby caused to rapidly evaporate, § 40 and 139a. This last named result is almost more surprising than the phosphorescent glow excited by molecular impacts on bodies not rendered perceptibly phosphorescent by light, § 55, at centre. Both phenomena will usually be found very telling in respect to the molecular constitution of matter and origination of thermal radiation, whether visible as light or not. In the whole train of Crookes investigations on the radiometer, the viscosity of gases at high exhaustion, and the electro-phenomena of high vacuums, ether seems to have nothing to do except the humble function of showing to our eye something of what the molecules and atoms are doing. The same confession of ignorance must be made with reference to the subject dealt with in the important researches of Schuster and J. J. Thomson on the passage of electricity through gases. Even in Thomson’s beautiful experiments, showing currents produced by circuital electro-magnetic induction in complete poleless circuits, the presence of molecules of residual gas or vapor seems to be the essential. It seems certainly true that without the molecules, electricity has no meaning. But in obedience to logic, I must withdraw one expression I have used. We must not imagine the “presence of molecules is the essential.” It is certainly an essential. Ether is certainly also an essential, and certainly has more to do than merely to telegraph to our eyes to tell us what the molecules and atoms are about. If the first step towards understanding the relations between ether and ponderable matter is to be made it seems to me that the most hopeful foundation for it is knowledge derived from experiment on electricity in high vacuum; and if, as I believe is true there is good reason for hoping to see this step made, we owe a debt of gratitude to the able and persevering workers of the last 40 years who have given us the knowledge we have; and we may hope for more and more from some of themselves and from others encouraged by the fruitfulness of their labors to persevere in the work.”
61b. Thomson’s Experiment. Velocity of Cathode Rays. 47The Elect., Lon., Oct. 5, ’94, p. 762; Phil. Mag., ’94.—The object of the experiment of J. J. Thomson was to determine whether the velocity approached that of light or that of molecules. The apparatus he employed involved the rotating mirror, which was fully described in Proc. Royal So., ’90, slightly modified. The rays were caused to produce phosphorescence, while the mirror was so adjusted that when at rest, the two images on the phosphorescent strips appeared in the same rectilinear line. Many other elements comprised the apparatus. All the steps were performed carefully and according to the best methods, but the results are those which in this experiment are of particular interest, for by knowing the velocity of the rays, their nature is better appreciated and that of the X-rays can be better deduced. The velocity bore a close relation to that of the mean square of the molecules of gases at temperatures zero ° C. or in the case of hydrogen, 1.8 × 105 cm. per second. As compared with such a velocity, that of the cathode rays was found to be in the neighborhood of 100 times as great, and this agrees very nearly with the velocity of a negatively electrified atom of hydrogen acquired under the influence of the potential fall, which occurred at the cathode. In further evidence of the verity of this statement, he made a rough calculation upon the curve or displacement produced by a magnet upon the rays. § 59. He stated: “The action of a magnetic force in deflecting the rays shows, assuming that the deflection is due to the action of a magnet on a moving electrified body, that the velocity of the atom must be at least of the order we have found.”
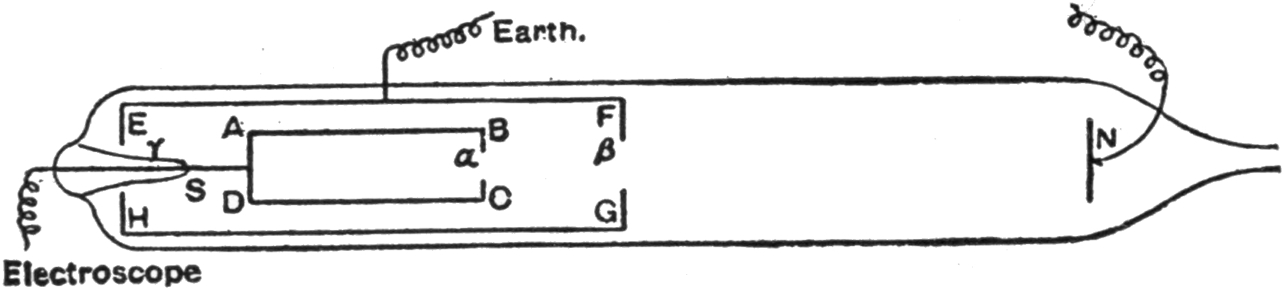
Fig. 1.
61b. Perrin’s Experiment. Cathode Rays Charged With Negative Electricity. Corresponding Positive Charges Propagated in the Reverse Direction and Precipitated upon the Cathode. Comptes Rendus, CXXI., No. 20, p. 1130; The Elect., Lon., Feb. 14, ’96, p. 523.—Jean Perrin’s object was to discover whether or not internal “Cathode rays were charged with negative electricity.” That they were had often been assumed by others, namely, Prof. J. J. Thomson, who considered cathode rays as due to negatively charged matter moving at high speed. § 61b. Again, Prof. Crookes, principally, and others, showed that they were possessed of mechanical properties and that they were deflected by a magnet. § 59. Perrin called attention to the above investigations and also alluded to the theoretical considerations of Goldstein, Hertz and Lenard, who favored the analogy of cathode rays to light—whose phenomena are well answered by the accepted theory concerning assumed etherial vibrations, which, in both cases, have rectilinear 48propagation, § 57, excite phosphorescence, § 54 and 55, and produce chemical action upon photographic plates. Great ingenuity was displayed, as might be expected, in the manner in which Jean Perrin proved the proposition named in the title of this section, at the Laboratory of the École Normale and also in M. Pallet’s Laboratory. First, therefore, let the elements of the discharge tube be thoroughly understood. As usual, the disk N is the cathode, referring to accompanying Fig. 1. A, B, C, D, is a metal cylinder having a small opening at the right hand end toward the cathode. Concentrically, is a similar cylinder, acting as an electrical screen and having a like opening similarly located as indicated. It corresponds to and plays the part of the Faraday cylinder, being connected to earth. The principle involved in this apparatus was based upon the laws of influence, which permitted him to ascertain the introduction of electric charges within a conducting envelope, and to measure such charges. During the discharge, the cathode rays were propagated from the cathode to and within the cylinder A, B, C, D, which immediately and invariably became charged with negative electricity. To prove that the charge was due to the cathode rays, he deflected them away from the opening in the protecting cylinder E, F, G, H. The cylinder was not under these circumstances charged, the rays being outside. He went further and made some quantitative analysis in a rough way to begin with. He related: “I may give an idea of the amount of the charges obtained when I state that with one of my tubes, at a pressure of .001 m. of mercury, and for a single interruption of the primary coil, the cylinder A, B, C, D, received sufficient electricity to bring a capacity of 600 C. G. S. units to a potential of 300 volts.” Upon the principle of the conservation of energy, he was induced, he said, to search for corresponding positive charges. “I believe I have found them in the very region where the cathode rays are generated, and that they travel in the reverse direction and precipitate themselves on to the cathode.” He verified this corollary by means of a modified feature of the apparatus shown in Fig. 2. The construction was 49the same except that there was a diaphragm having a perforation β´ within the protecting cylinder and opposite the smaller cylinder exactly as indicated, so that the positive electricity which had entered through β could only act on the cylinder A, B, C, D, by traversing also the hole β´. “When N was the cathode, the rays emitted traversed the two apertures at β and β´ without any difficulty, and caused the gold leaves of the electroscope to diverge widely. But when the protecting cylinder was the cathode, the positive flux, which, as was shown by a previous experiment, enters by the aperture β, did not succeed in separating the gold leaves, except at very low pressures. If we substitute an electrometer for the electroscope we shall see that the action of the positive flux is real, but that it is very small and increases as the pressure decreases.”

Fig. 2.
He inferred that: “These results, taken as a whole, do not appear to be easily reconcilable with the theory that the cathode rays are ultra-violet light. On the contrary, they support the theory that attributes these rays to radiant matter, § 54, near centre, a theory, which may at present, it seems to me, be enunciated as follows: In the vicinity of the cathode the electric field is sufficiently strong to tear asunder into ions some of the molecules of the residual gas. The negative ions start off toward the region where the potential increases, acquire a considerable velocity, and form cathode rays; their electric charge, and consequently their mass (at the rate of one gramme equivalent per 100,000 coulombs) is easily measured. The positive ions move in the reverse direction; they form a diffused tuft, susceptible to magnetism, but are not a regular radiation.”
61c. Zeugen. Comptes Rendus, Jan. 27, 1896.—In a note regarding the experiments of Roentgen, called attention to his own communications to the Academie des Sciences in February and August 1886, describing his photographs of Mt. Blanc taken in the night by the invisible ultra-violet rays. This note is entered as many newspapers reported the photograph to be due to cathode rays, imagine the intense phosphorescence upon a screen at the top of the mountain, if such were the case.
62. Goldstein’s Experiment. Phosphorescence of Particular Chemicals by Cathode Rays. Nature, Lon. Feb. 21, ’95, 50p. 406. Weid. Ann., No. II, ’95.—Lithium chloride when acted upon by cathode rays, phosphoresced to a dark violet color or heliotrope, which it retained for some time in a sealed tube. Chlorides generally and other haloid salts of potassium and sodium showed similar effects. The colors were superficial and could be driven away rapidly either by heating or the action of moisture.
63. Kirn’s Experiment. Spectrum of Post Phosphorescence of Discharge Tubes. Wied. Ann., May, ’94. Nature, Lon. June 7, ’94, p. 131.—Carl Kirn compared the spectra of the phosphorescence of a vacuum bulb, during and immediately after the discharge. The details are as follows: The spectrum of the after-glow, § 54, at end and 22, was found to be continuous. In this connection, see a plate showing different kinds of spectra, for example, Ganot’s Physics, frontispiece. The spectrum shortened from both directions to a band between the wave lengths of 555 and 495µµ. The spectrum then continued to grow shorter and shorter until it disappeared at the line E, which is the position of the greatest luminosity of the solar spectrum. For experiments on spectrum, see Fraunhofer in Gilbert’s Ann., LVI. During the discharge, the spectroscope showed a line spectrum corresponding very closely to those of carbonic acid gas and nitrogen. Some authorities had suggested that perhaps the after phosphorescence and the beginning of the incandescence of a solid body, were the same kind of light, but this experiment shows that such is not the case, unless some relation exists on the ground that the two phenomena are exactly opposite to each other, and it confirms similar results obtained by Morrin and Riess. The result indicates that the nature of the phenomenon is not identical in all respects with light produced at a high temperature.
63a. De Metz’s Experiment. Chemical Action in the Interior of the Discharge Tube. Internal Cathode Rays. L’Ind. Eler., May 10, ’96, and Comptes Rendus, about April, ’96. Translated by Louis M. Pignolet. He used a cylindrical discharge tube divided into two halves which fitted together by an air-tight ground joint. In one-half were the anode and the cathode; in the other half was the holder containing the sensitive paper or films. The holder was exposed to the direct action of the cathode rays and was closed by a cover of cardboard or sheet aluminum. The objects to be photographed were placed between the cover and the sensitive film or paper. The tube was connected to a Sprengel pump which maintained its vacuum during the experiments. In this way, twelve photographs were 51taken from which it appeared that cathode rays, like X-rays, penetrate cardboard and aluminum, but are stopped by copper (1.26 mm.) and platinum (0.32 mm.). Poincaré, in a note in the same publications as the foregoing, criticised the results of the experiments of De Metz, claiming they did not prove irrefutably that cathode rays possessed the essential properties of X-rays, for the cathode rays in impinging on the cover of the holder would generate X-rays, § 91, which would give the results obtained. Poincaré did not deny the fact.
63b. Hertz’s Experiment. The Passage of Cathode Rays Through Thin Metal Plates Within the Discharge Tube. Diffusion. Wied. Ann., N. F. 45; 28, 1892. Contributed by request, by Mr. N. D. C. Hodges of the Hodges Scientific News Agency, N.Y. Found in records at Astor Library.—A piece of uranium glass was covered partly on one side (which he calls the front side) with gold-leaf, and on the gold leaf were attached several pieces of mica. This front side was then exposed to cathode rays. So long as the exhaustion had not proceeded far, and the cathode rays filled the whole tube with a blue cone of light, only the portion of the uranium glass outside the gold-leaf screen showed any phosphorescence. But as soon as the exhaustion had progressed far enough, and the light began to disappear, the genuine cathode rays struck the covered glass, and the phosphorescence manifested itself behind the gold-leaf. When the cathode rays were fully developed, the gold-leaf hardly had any effect, while the mica cast deep black shadows. The same experiment was tried with silver-leaf, aluminum and alloys of tin, zinc and copper. Aluminum showed the best results; sheets which allowed no light to pass, allowing the cathode rays free passage. The rays after their passage through the metal screens did not continue their straight course, but seemed to be diffused much as light is diffused by passing through a cloudy medium. In this connection reference is made to the work of Goldstein, who had noticed also the reflection of “electric” rays. Wied. Ann., N. F. 15; 246, 1882. In 1893, Goldstein published further accounts concerning actions in discharge tube. Wied. Ann., vol. 48, p. 785.
65. Lenard’s Experiments. Cathode Rays Outside of the Discharge Tube. Wied. Ann., Jan., ’94, Vol. LVI., p. 225; The Elect., Lon., Mar. 23 and 30, ’94, Apr. 6, ’94; and Elect. Rev., Lon., Jan. 24, ’96, p. 99.—Of more importance in connection with X-rays is the consideration of Lenard’s experiments than any others. The reader must bear in mind that his exhaustive investigations resulted from his discovery (founded upon a hint from Hertz) that the cathode rays might be transmitted to the outside of the generating discharge tube. His interest, therefore, in the discovery was so great that his researches extended to the minutest details. Passing from these introductory remarks, the characteristics of the tube that he employed will be explained first. Reference may now be made to the accompanying Fig. A. He employed several different kinds of tubes, but finally settled upon one of which the essential elements are shown in the said figures. It was permanently connected to the pump, § 53, so that the pressure within could be varied. Opposite the cathode, which consisted of a thin disk of aluminum, the end of the tube was provided with a thick metal cap, having a perforation, which in turn was closed by a thin aluminum sheet secured by marine glue in an air-tight manner, and called a window. The anode was a heavy brass cylinder, shown in section, within the discharge tube and surrounding the leading-in wire of the cathode. The anode and the aluminum window were connected to each other, electrically, and to earth, as well as two a secondary terminal of an induction coil, whose electrodes were in shunt to those of the discharge tube, in order that the operator might adjust the sparking distance which rapidly increased with the exhaustion. The induction coil had a mercury interrupter.
65. Properties of Cathode Rays in Open Air.—In all directions around the window upon the outside and in the open air, a faint bluish glow (§ 11 and 140) extended and vanished at a distance of 5 cm., as indicated by dotted lines in Fig. B at beginning 54of this chapter. The degree of luminosity may be judged by saying that it was not sufficient to admit of investigation by the ordinary pocket spectroscope. A new window was void of luminosity; but with use, bluish gray and green and yellow spots occurred thereon.
66. Phosphorescence by Cathode Rays.—Substances which generally phosphoresced by light and cathode rays in the generating bulb, § 55, also phosphoresced under the influence of the rays in open air, excepting eosin, gelatin, both phosphorescent in light, were not so in cathode rays; so also with solutions of fluorescein, magdala red, sulphate of quinine and chlorophyll. Phosphorescence was less if the rays first passed through a tube of glass or tinfoil lengthwise. The phosphorescent light of the phosphides of the alkaline group, uranium glass, calcspar and some other substances, was so great that the luminosity of the air was invisible by contrast. The maximum distance at which phosphorescense was discernable in open air was about 8 cm. The best phosphorescent screen consisted of paper saturated with pentadecylparatatolylketone. In order to prepare it, he laid a sheet of paper upon glass and applied the fused chemical with a brush. As to the color of the phosphorescence and fluorescence of different substances, and as to the degree of luminosity outside of the vacuum tube, they were about the same as reported by Crookes when located within the discharge tube. §55. Baric and potassic and other double cyanides of platinum, common flint, glass, chalk and asaron all exhibited the same property as when exposed to ultra-violet light, that is, fluoresced or phosphoresced. Sulphide of quinine in the solid state fluoresced, but not in solution. Petroleum spread on a piece of wood fluoresced, and also fluorescent-hydrocarbons generally.
66a. The cathode rays were not easily transmitted by tinfoil or glass, because the degree of phosphorescence on the screen was greatly reduced by interposing such sheets. The phosphorescense ceased also by deflecting internal cathode rays from the window by a magnet. For full treatment of the phenomena of phosphorescence, see Stokes’ experiments, described in Phil. Trans., 1852, Art. “Change of Refrangibility of Light.” In brief, Stokes’ theory assumes that such substances have the power of reducing the refrangibility. Example: Ultra-violet light, highly refractive, is changed to yellowish green, less refrangible, by reflection from uranium glass.
67. The Aluminum Window, a Diffuser of Cathode Rays. § 63b. The conclusion arrived at by mounting the phosphorescent screen in different positions and at different angles as well 55as by observance of the gaseous luminosity, was that the aluminum window scattered the rectilinear parallel cathode rays in all directions, § 57.
68. Transmission of External Cathode Rays Through Metals.—The phosphorescence was not diminished apparently by an intervening gold-leaf or silver or aluminum foil, while it was extinguished by quartz .5 mm. thick which also cut off the atmospheric glow beyond itself. The leaves and foil did not so act. The difference of thickness should be borne in mind, as metal, as thick as the quartz did not transmit. As to other substances, tissue paper cast a slight shadow, which was darker with an additional sheet; but the shadow was independent of color and blackness, § 154. Ordinary writing paper was roughly, proportionally opaque, while the shadow was black with cardboard .3 mm. thick. Glass films as made by blowing glass, cast faint shadows when .01 mm. thick. He proved that there was little difference as to the transmitting power of conductors and dielectrics when thin. Mica and collodion sheets .01 mm. thick cast scarcely any shadow. The reader may bear in mind the striking differences between these properties of cathode rays, and X-rays, § 135, it being assumed always that the generating devices are the same; for example, water permitted the cathode rays (were these simply feeble X-rays?) to be transmitted only when in very thin layers. Even soap water films which were only .0012 mm. thick cast shadows, although very faintly. The shadows of drops of water were black, while water several feet thick has been traversed by X-rays from a small set of apparatus. By careful measurements he found that the law of transmission must be different from that of light, for in the latter, many substances are opaque although exceedingly thin, while with cathode rays, the same will traverse all films. Goldstein and Crookes reported that thin mica, glass and collodion films made very dark shadows, § 58, within the discharge tube, whereas Lenard found that outside of the vacuum tube, in open air, the transparency was greater than according to the earlier experimenters, but he acknowledged that Crookes and Goldstein were inconvenienced and limited in the number of observations because it is so difficult to carry on such experiments within an hermetically sealed tube. Again, he acknowledged that perhaps the cathode rays of those experimenters were of a different kind. The construction shown in the above figures was modified by using a very thin glass window instead of aluminum, and the results were the same allowing for the different opacity, to ordinary light, of aluminum and glass.
56The cathode rays acted upon the sense of smell and taste as the nose and mouth could detect ozone, § 84, at end.
69. Propagation. Turbidity of Air. Upon studying the shadows on the phosphorescent screen, it was noticed that the rays were bent around the edges of the object. Again, when the object had a slit, diffusion could be noticed by the shape (as in Crookes Ex., Fig. 15, p. 17,) of the luminous portion of the phosphorescent screen. In Fig. B, at beginning of this chapter, the spatter work represents the shape of the luminous portion, the darker part representing the most luminous surface of the screen, the latter being held at right angles to the thick plate, having the slit and opposite the aluminum window. By varying these experiments, especially by changing the angle of the screen he found that not the all rays were diffused, but as in the passage of light through milk, some were transmitted in rectilinear lines.
70. Photographic Action.—He performed with sensitive silver compound papers, an experiment somewhat similar to those with phosphorescent bodies and also others. Behind a rather thick opaque plate the chemical film was not acted upon, but the rate of blackening near the aluminum window without obstruction of intermediate bodies was about the same as that with befogged sunlight. The former, moreover, was acted upon at a much greater distance than that at which phosphorescence was exhibited and beyond the atmospheric luminosity. By means of shadow pictures or sciagraphs, he compared the shadows produced by the external cathode rays with those which would have been obtained by light. Referring to Fig. C, beginning of this chapter, the sensitive plate was half covered with a plate of quartz, Q, and half with a plate of aluminum, A´ overlapping the quartz. With light, the shadows would have appeared as in said figure, that is, one-half black as produced by aluminum, a quarter rather light as produced by quartz, and the other quarter bright, or a similar arrangement, according to whether the negative or the positive photograph is considered; but with the cathode rays, the appearance of the developed plate was as in Fig. D., beginning of this chapter. The quartz cast the black shadow, while the aluminum, the lighter one. Furthermore, the luminosity of the air produced a variable light on the other quarters. A similar appearance was produced by casting shadows of such plates upon the phosphorescent screen; but, of course, the picture was not a permanent one. The photographic plate served to accumulate the power, for the cardboard which cast a faint shadow upon the phosphorescent 57screen, showed a black shadow upon the photographic paper by sufficiently long exposure. At the same time, strips of thin metal were placed side by side between the chemical paper and the cardboard, and they showed different degrees of shading. The cardboard was quite thick, being .3 mm. Prof. Slaby (see Elect. Rev., Lon., Feb. 7, ’96), after Röntgen’s discovery, produced sciagraphs of the bones of the hand at the window of the Lenard tube. Lenard doubted whether the cathode rays produced direct chemical action. Iodine paper became bluish, but he could not obtain other chemical effects usually produced by light, and other agencies, for example, oxygen and hydrogen mixed together in the proportion to form water, and which were in their nascent state, and which were located in a soap-bubble, did not explode or ignite. No effect was produced upon carbon bi-sulphide nor hydrogen-sulphide, although the exposure was very long. Ammonia was not formed when the rays acted upon a mixture of three parts hydrogen and one part nitrogen, as to volume. He thought that he noticed a small expansion of air, hydrogen and carbonic acid separately located in a vessel having a capillary tube and water to indicate the expansion. He attributed the slight expansion to an indirect action, although very slight, caused by heat produced by the cathode rays, § 27, and yet neither the thermopile nor the thermometer showed any calorific effects although the thermopile responded to the flame of a candle 50 cm. distant.
71. Cathode Rays and Electric Forces Distinguished. The earth connection heretofore mentioned with the aluminum window was for the purpose of dispensing with sparking, but even then the approach of another conductor connected to earth would cause some sparking. Sparks could be drawn when the cathode rays were deflected from the aluminum window by a magnet. Fig. E, at beginning of chapter. He argued that the rays and the electric forces of the spark are non-identical. He was not satisfied with this as an absolute proof, and he instituted others. He enclosed the whole generator in a large metal box. In the observation space, that is, around and near the window, he located another box, having an aluminum front facing the window. See Fig. E, at beginning of chapter. It was within this second box that he took the sciagraph shown in Fig. D, at beginning of chapter. It is important to notice that sparks could not be drawn at points within the said second box, shown at the left, even by a metallic point shown projecting thereinto. No spark occurred whatever, not even from the aluminum front. Sparking occurred when the pointed wire was extended to a considerable 58distance outside of the back of the small box, but it was remarked that the electric force did not enter through the front wall but was introduced “from behind into the box, by the insulation of the wire.” No one can, therefore, enter the objection that the cathode rays experimented with, were generated from the aluminum window as a cathode. They came from the cathode referred to entirely within the vacuum tube. Prof. J. J. Thomson, F. R. S., had at an early date conjectured that cathode rays did not pass through thin films of metal, but that these films acted as intermediate cathodes themselves. See his book on “Recent Researches,” p. 26, also The Elect., Lon. March 23, ’94, p. 573, in an article by Prof. Fitzgerald, who names that citation.
72. Cathode Rays Propagated, but not Generated in a High Vacuum.—The proposition was proved by having two tubes, one called the generating tube and one the observation tube, the former being like that shown in Fig. A, at beginning of chapter, which is partly repeated in Fig. F, at beginning of chapter, combined with the observation tube, which contains the two electrodes for casual use; but the one on the right is a disk extending nearly throughout the cross sectional area, and having a small central opening. Although both tubes were connected to the air pump, yet, by means of stop-cocks, the vacuum in one tube could be maintained at a maximum degree for hours, while the other was at a minimum. The first experiment was performed with a vacuum, about as high as that employed in Crookes’ phosphorescent experiments, § 53. There was a patch of green light, § 57, at the extreme left end of the observation tube and the glass was green at the right, § 54, and a little to the left of the perforated disk electrode a. The other electrode of this tube was located at the upper left and lettered k.
72a. The magnet deflected the rays in the observing tube as indicated by the partial extinction of the phosphorescent patch. He noticed that with the rarefied atmosphere the amount of turbidity was enormously reduced, or in other words, that the rays were propagated more nearly in rectilinear lines. All the experiments on the cathode rays, in this observing tube, were of about the same nature as those which could be produced in the discharge tube.

From Sciagraph of Cat’s Leg, by Prof. William F. Magie.
Copyright, 1896, by William Beverly Harison, pub. of X-ray pictures, 59 Fifth Ave., New York City.
72b. The principal experiment consisted in exhausting the observing tube to such a degree that cathode rays could not be generated therein. The vacuum was so perfect that when used as a discharge tube all phosphorescence gradually died away until it disappeared, and no current passed (§ 25) except on the outside surface of the glass. The coil was so large, electrically, 60that the length of the spark between spheres was 15 cm. Upon charging the right hand tube and generating cathode rays, it was determined by means of magnetic deflection, phosphorescence and other effects, that the cathode rays traversed the highest possible vacuum (§ 19, near end, where energy must have passed through the high vacuum to produce luminosity in the inner bulb). The external and internal rays were certainly different forms of energy. Inasmuch as he noticed that rarefied air was less turbid and less absorptive than air at ordinary pressures, it occurred to him to make a very long tube, namely, 1 m, or a little over 3 feet. He employed very severe steps for obtaining an exceedingly high vacuum, the operation occupying several days. The pump used was a Toepler-Hagen, while a Geissler pump was employed separately for the discharge tube. The pencil of cathode rays traversed the whole length of the long tube. See a portion of the apparatus in Fig. G, at beginning of this chapter. One disk was of metal and perforated with a pin hole and the other was a phosphorescent screen, so that when the cathode pencil passed through the hole in the plate a patch was seen upon the phosphorescent screen. The phosphorescent spot was always, no matter what the relative distances of the disks were from each other, and from the end of the tube, substantially the same as it would have been by calculation assuming that there was no turbidity effect. The patches, in each instance, were a little smaller in diameter than the calculated ones. For example with one measurement, at certain distances, the actual diameter of the patch was 2.5 mm., while the calculated diameter was 2.9 mm. In his experiments with light under the same conditions, the luminous spots were also a little smaller than the calculated or geometrical. The disks had iron shoes and were moved to different positions by a magnet. He concluded, therefore, that in what may be called a perfect vacuum, light and cathode rays have a common medium of propagation, namely, the assumed ether. Prof. Fitzgerald, in The Elect., Lon. Mar. 23, ’94, does not agree broadly with him in this; neither does he contradict him. He argues rather on the point that the cathode rays and light rays are not identical, but Lenard does not affirm this, because the magnet will attract the former and not the other. Prof. Fitzgerald admits this and calls to mind that even in a vacuum, as obtained by Lenard, there were still ten thousand million molecules per cu. mm. and therefore he thinks it is better to look to matter rather than ether as the medium of propagation of cathode rays. § 61b. On the other hand, Lenard agrees with certain other predecessors, 61Wiedemann, Hertz and Goldstein, in favor of cathode rays being etheric phenomena. See Wied. Ann., IX., p. 159, ’80; X., p. 251, ’80, XII., p. 264, ’81; XIX., p. 816, ’83; XX., p. 781, ’83. The vacuum with which Lenard operated, was .00002 mm. pressure, obtained by cooling down the mercury to minus 21° C. This vacuum was so high that all attempts to prove the presence of matter failed. Neither did the exceedingly high vacuum deaden the cathode rays. On the other hand, as noted, they were assisted rather than hindered. § 135.
73. Cathode Rays. Phenomena in Different Gases.—The apparatus consisted of an observing tube having a tubular gas inlet and outlet both in one end and arranged in line with the cathode of the discharge tube. See construction in Fig. H, at beginning of this chapter, the tube being about 40 cm. long and 3 cm. in diameter. He was very careful in every case to chemically purify and dry the particular gas. He omitted the perforated disk and provided an opaque strip of the phosphorescent screen on the side toward the window and made his observations from the other side, the object of the experiment being particularly to test the transmission of cathode rays in different gases. With any particular gas, he moved the phosphorescent screen along by means of a magnet until the shadow on the screen became invisible. It is evident that the distances of the screen from the window for different gases would indicate the relative transmitting powers. He also modified the experiment by varying the density of the gases, hydrogen being taken as 1 as usual, nitrogen 14, and so on. The transmitting power of hydrogen was nearly five times as great as that of nitrogen, air, oxygen and carbonic acid gas, which did not much differ. § 10 and 18. Sulphurous acid was a very weak transmitter. All the gases became luminous near the window as in air. § 65. The colors were all about the same as far as distinguishable, § 11, which was difficult in view of the brightness of the phosphorescence on the glass. It was a universal rule, that when the density decreased, the transmitting power increased. In high vacua, in all gases, the rays went through the space in rectilinear lines in all directions from the window, and generally it made no difference what gas was employed provided the vacuum was as high as hundredths of a millimetre. At this pressure all gases acted the same. To be sure, the phosphorescence did not occur at this high vacuum at a great distance as might be expected, but it should be remembered that the intensity of the rays varied as the square of the distance, and, therefore, at very great distances, the action was very weak.
6274. Cause of Luminosity of Gas Outside the Discharge Tube.—At ordinary pressures, in the cases of hydrogen and air, as has been noted, the gas became luminous in the observing tube, the effect being, of course, the same as entering open air, represented in Fig. A, beginning of this chapter. In order to determine the luminosity at less pressures, the gas, of whichever kind, was enclosed in a rather long observing tube and only at rather high vacua did the bluish and sometimes reddish gaseous luminosity disappear. Upon grasping the tube with the hand or approaching any conductor connected to earth, of large capacity, the column stopped at that point so that the remainder of the tube, beyond the hand, measured from the discharge, was dark. The phosphorescence on the glass wall of the tube produced by the cathode rays was not influenced in any way by outside conductors, such as the hand. Cathode rays themselves were not stopped apparently by the hand, because the phosphorescent screen and glass, located beyond the hand, became luminous. He concluded, therefore, that the glowing of the gas had no close connection with the cathode rays. He proved this also by deflecting the cathode rays in the discharge tube from a certain space, and yet the gaseous luminosity remained. As an exception, the cathode rays sometimes appeared to be closely associated with the light column. He attributed the luminosity of the gas in general, at low pressures, not to the cathode rays, but directly to the electric current or some kind of electric force, § 11 and 14, which, as already remarked, permitted sparks to be drawn from the aluminum window and surrounding points.
The negative glow light in Geissler tubes, § 30, is also to be regarded as gas illuminated by cathode rays. (Compare Hertz, Wied. Ann., XIX., p. 807, ’83.) Between that phenomenon and the glow observed here and attributed to irradiation, there exists a correspondence, inasmuch as in both cases the light disappears at high exhaustions, § 53, appears fainter and larger when the pressure increases, § 54, and then becomes brighter and smaller, § 54. But, whereas, the glow in the Geissler tube has become very bright and small at 0.5 mm. pressure, the gas in our experiment remains much darker up to 760 mm. pressure, and yet the illuminated spot is much larger. This difference cannot, therefore, be attributed to an inferior intensity of the rays here used. But it will be explained, § 76, as soon as we can show that at higher pressures cathode rays of a different kind are produced, which are much more strongly absorbed by 63gases than the rays investigated hitherto and produced at very low pressures.
Fig. I, p. 52, illustrates the apparatus by which he studied the rectilinear propagation and whereby he found that it was rectilinear only in a very high vacuum. In the figure, the gas is at ordinary pressure, and it will be noticed that the turbidity of the same is indicated by the curved lines while the dotted lines show the volume that would be occupied by light or other rectilinear rays, unaccompanied by any kind of diffusion. In the observing tube, there was a disc having a central hole at a. Beyond this disc, measured from the aluminum window, was a fluorescent screen which, as well as the perforated disc, could be moved to different distances by means of a magnet acting on a little iron base. It is evident that upon moving the fluorescent screen to different distances, the diameter of the luminous patch would be a measure of the amount of turbidity. The curved lines intersecting the peripheries of the luminous spots indicate, therefore, the field of the cathode rays, so that said field would appear like a kind of curved cone if the same were visible. Although hydrogen is the least turbid gas, yet the phosphorescent patches were all larger except with a high vacuum than they could have been with rectilinear propagation. An additional characteristic of the phosphorescent spot, was its being made up of a central bright spot and a halo less luminous, appearing like some of the pictures of a nebula, see Fig. I´, p. 52, the darker or centre indicating the brighter portion. In a perfect vacuum the halo did not exist. He performed a similar experiment with ordinary light. No halo occurred on a paper screen which was used instead of the phosphorescent screen, but upon introducing a glass trough of dilute milk between the window and the perforated disc, or between the disc and the paper screen, nuclei and halos were obtained, illustrating a case of the effect of a turbid fluid upon light, and assisting in proving that gases act as a turbid medium to cathode rays as milk and similar substances do to light; also in other gases than hydrogen, and by the use of cathode rays, nuclei and halos were not obtained at high exhaustion, all the gases becoming limpid. Taking into account pressure and density, all gases behaved the same as to the power of transmission when they were of the same density, without any regard whatever to their chemical nature. Density alone determined the matter, according to Lenard.
75. Cathode Rays of Different Kinds are Variably Diffused.—He discovered the remarkable property, contrary to his 65expectation, that if the rays are generated at high pressures, they are capable of more diffusion than when generated at lower pressures. This can be easily proved by any one, for it will be noticed that upon increasing the pressure in the discharge tubes the spots on the phosphorescent screen will not only grow darker but larger and more indefinite as to the nucleus and halo. He called attention to the agreement with Hertz, who also found that there were two different kinds of rays, see Wied. Ann., XIX, p. 816, ’83, also see Hertz’s experiment. Lenard also pointed out the analogue in respect to light, which, when of short wave length, is more diffused in certain turbid media than that of greater wave length. Although Lenard held that his experiment proved that cathode rays were phenomena in some way connected with the ether, yet he pointed out an important difference in connection with the property of deflection of the rays by the molecules even of elementary gases like hydrogen, producing diffusion of the rays, which accordingly may be considered as behaving like light in passing through, not gases, but vapors, liquids and dust. In the case of the cathode rays the molecules of a gas acted as a turbid medium, but in the case of light, turbidity is only exhibited by vapors or certain liquids, as so eloquently explained by Tyndall, in “Fragments of Science,” 1871, where it is shown that aggregation of molecules, like vapors or dust in the presence of light, make themselves known by color and diffusion, whereas the substances in a molecular or atomic state do not serve to show the presence of rays of light.
76. Law of Propagation.—Lenard recognized continually that there were two kinds of cathode rays. One of them may have been X-rays without his knowing it. In the latter part of ’95, he made some experiments especially of a quantitative nature as to the principle of absorption of the rays by gases. By mathematical analysis, based upon experiments, he arrived at the principle that the absorptivity of a gas is proportional to its pressure, or what is the same thing, to its density, or as to another way of stating the law, “the same mass of gas absorbs at all pressures the same quantity of cathode rays.” See Elect. Rev., Lon., as cited, p. 100.
77. Charged Bodies Discharged by Cathode Rays.—An insulated metallic plate was charged first with positive electricity and in another experiment with negative electricity. In each instance, the plate was discharged rapidly by the cathode rays as indicated by the electroscope, and the same held true when a wire cage in contact with the aluminum window, surrounded the electroscope and the metallic plate. The effect was 66stopped by cutting off the cathode rays by quartz .5 mm. thick. The discharge took place, however, through aluminum foil. A magnet was made to deflect the internal cathode rays, whereupon the discharge did not take place, all showing that the discharge of the insulated plate was directly due to those rays. A remarkable occurrence was the accomplishment of the discharge at a much greater distance than that at which phosphorescence was exhibited. See also Roentgen’s experiment—who suggested that Lenard had to do with X-rays in this experiment, but thought they were cathode rays. The maximum distance for the discharge was about 30 cm. measured normally to the aluminum window. He caused a discharge of a plate also in rarefied air. He admitted that the experiments were not carried far enough to know whether the effect was due to the action of the cathode rays upon the surface of the window, or upon the surrounding air, or upon the plate. The author could not find in Lenard’s paper any positive or negative proof that he had actually deflected the external cathode rays by a magnet while passing through air or gas at ordinary pressure. He had deflected them while passing through a very high vacuum in the observing tube. Dr. Lodge, who briefly reviewed Lenard’s experiments, expressed the same opinion. See The Elect., Lon., Jan. 31, ’96, p. 439. For theoretical considerations of the electric nature of light, the discharge law in the photo-electric phenomena, the simple validity of the discharge law, the occurrence of interference surfaces in the blue cathode light, the cathode rays in the axis of symmetry, the necessary degrees of longitudinal electric waves, the frequency of the cathode rays, and proof of longitudinal character of cathode rays, see Jaumann in The Elect., Lon., Mar. 6, ’96; translated from Wied. Ann., 571, pp. 147 to 184, ’96, and succeeding numbers of The Elect., Lon., which were freely discussed in foreign literature contemporaneously.
78. De Kowalskie’s Experiment. Source, Propagation and Direction of Cathode Rays. Acad. Sci., Paris, Jan. 14, ’95; So. Fran. Phys. Jan. ’95; Nature, Lon. Jan. 24, ’95; Feb. 21, ’95.—The conclusions he arrived at are, 1. The production of the cathode rays does not depend on the discharge from metallic electrodes across a rarefied gas, nor is their production connected with the disintegration of metallic electrodes. 2. They are produced chiefly where the primary illumination attains suitable intensity, that is, where the density of the current lines is very considerable. 3. Their direction of propagation is that of the current lines at the place where the rays are produced, from the negative to the positive poles. They are propagated 67in the opposite direction to that in which the positive luminosity is supposed to flow. § 43. He employed a Goldstein tube reduced at the centre. § 41. It was found that the cathode rays are formed not only at the negative electrode, but also at the constriction, directly opposite the cathode. De Kowalskie carried on further experiments in this line in order to be satisfied with the principles named above, which he formulated. In one tube, he was able to produce cathode rays at either end of the capillary tube forming the constricted part of a long vacuum tube. No electrodes were employed. The tube was merely placed near a discharger through which “Tesla currents” were passed? He seems to have been working with X-rays without knowing it; for his results agree with those of Roentgen and later experimenters that the source of X-rays is the surface of a substance where it is struck by cathode rays. The statements were about as definite as could be expected at that date.
79. Roentgen’s experiments. X-Rays, and A New Art. Wurz. Physik. Med. Gesell. Jan. ’95; Nature, Lon., Jan. ’96; The Elect., Lon. April 24, 96; Sitz. Wurz. Physik. Inst. D. Uni. Mar. 9, 96.—Uninfluenced By A Magnet In Open Air.—Although Lenard recognized several kinds of cathode rays, which differed as to penetrating and phosphorescing power, yet he always held, or inferred at least that they were deflected by a magnet, outside, as well as inside, (proved § 72a.) of the discharge tube. § 59. Prof. Wilhelm Konrad Roentgen subjected his newly discovered rays to the action of very strong magnetic fields in the open air, but no deviation was detected. This is the characteristic which more than anything else has served to distinguish X-rays from cathode rays. This property has been confirmed by others. He employed the principle of magnetic attraction of internal cathode § 59, rays to shift the phosphorescent spot, for then he noticed that the source of X-rays fluctuated also.
80. Source of X-Rays may Be At Points Within The Vacuum Space.—In one case, he employed a Lenard tube, and found that the X-rays were generated from the window which was in the path of the cathode rays. § 67. Different bodies within the discharge tube were found to have different quantitative powers of radiating X-rays when struck by the cathode rays. He stated “If for example, we let the cathode rays fall on a plate, one half consisting of a 0.3 mm. sheet of platinum and the other half a 1 mm. sheet of aluminum, the pin-hole photograph of this double plate will show that the sheet of platinum emits a far greater number of X-rays than does the aluminum, this remark applying in every case to the side upon which the cathode rays impinge.” On the reverse side, however, of the platinum, no rays were emitted, but a large amount was radiated from the reverse side of the aluminum. § 67. He admitted that the explanation was simple; but, at the same time, he pointed out that this, together with other experiments, showed that platinum is the best for generating the most powerful X-rays. 70One form with which he experimented is illustrated in Fig. J, in principle, being described as a bulb in which a concave cathode was opposite a sheet of platinum, placed at an angle of 45° to the axis of the curved cathode, and at the focus thereof.
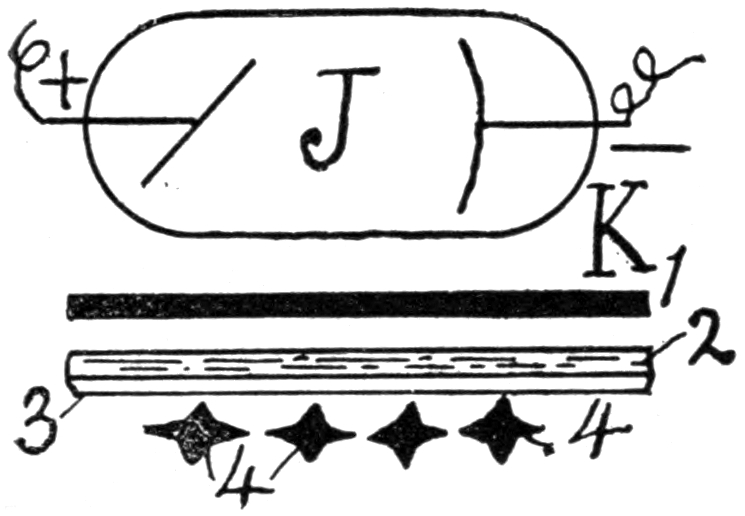
J
81. Reflection of X-Rays.—He emphasized the knowledge that there is a certain kind and a certain amount of reflection, such as that produced upon light and, as pointed out by Lenard, upon cathode rays, by certain turbid media. The following quotation sets forth the exact experiment to show slight reflection at metal surfaces. “I exposed a plate, protected by a black paper sheet 1 to the X-rays (e.g. from bulb J) so that the glass side 2 lay next to the discharge tube. The sensitive film was partly covered with star-shaped pieces (4 slightly displaced in the Fig.) of platinum, lead, zinc and aluminum. On the developed negative the star-shaped impressions showed dark (comparatively) under platinum, lead and more markedly, under zinc; the aluminum gave no image. It seems, therefore, that the former three metals can reflect the X-rays; as, however, another explanation is possible, I repeated the experiment with only this difference, that a film of thin aluminum foil was interposed between the sensitive film and the metal stars. Such an aluminum plate is opaque to the ultra-violet rays, but transparent to X-rays. In the result the images appeared as before, this pointing still to the existence of reflection at metal surfaces.”
82. Penetrating Power. The transmitted energy was tested both by a fluorescent screen and by a sensitive photographic plate. Either one was acted upon by the rays after transmission through what have ordinarily been called opaque objects. § 68. for example, 1000 pages of a book. As in Lenard’s results, so in Roentgen’s, the color of the object had no effect, even when the material was black. § 68, near beginning. A single thickness of tinfoil scarcely cast a shadow on the screen. § 66a. The same was true with reference to a pine board 2 or 3 cm. thick. They passed also through aluminum 15 mm. thick. 63b. Glass was comparatively opaque, § 66a, as compared with its power of transmitting light, but nevertheless it must be remembered that the rays passed through considerable thickness of glass. The tissues of the body, water § 68, near centre, and certain other liquids and gases were found exceedingly permeable § 67. Fluorescence could be detected through platinum 2 mm. thick and lead 1.5 mm. thick. Through air the screen was illuminated at a maximum 71distance of 1 m. A rod of wood painted with white lead cast a great deal more shadow than without the paint, and in general, bones, salts of the metals, whether solid or in solution, metals themselves and minerals generally were among the most resisting materials. § 155. The experiments were performed in a dark room by excluding the luminosity of the tube by a thick cloth or card board entirely surrounding the tube. He performed the wonderful experiment, so often since repeated, of holding the hand between the screen of barium platino cyanide and the discharge tube, and beholding the shadow picture of the bones. This was the accidental step which initiated the new department of photography, and which gave to the whole science of electric discharge, a new interest among scientists and electricians and which thoroughly awakened popular interest. The whole world concedes to him the honor of being the originator of the new art. In view of sciagraphs of the bones of the hand upon the screen, it occurred to him in view also of Lenard’s experiments, on the photographic plate, to produce a permanent picture of the skeleton of the hand with the flesh faintly outlined. § 84. The accompanying half tone illustration, page 37, was made by the Elect. Eng. N.Y. (June 3, ’96) by permission, and it represents the Edison X-ray exhibit at the New York Electrical Exposition of the Electric Light Association, 1896. Thousands of people, through the beneficence of Dr. Edison, were permitted to see the shadows of their bones surrounded by living flesh. The screen was made of calcic tungstate. The hand and arm were placed behind and viewed from the front. § 132, near beginning.
83. Penetrating Power and Density of Substances.—Although he found that there was some general relation between the thickness of materials and the penetrating power, yet he was satisfied that the variation of the power did not bear a direct relation to the density, (referring to solids) especially as he noticed a peculiar result when shadows were cast by Iceland spar, glass, aluminum and quartz of equal thickness. The Iceland spar cast the least shadow upon suitable fluorescent or photographic plate. The increased thickness of any one substance increased the darkness of the shadow, as exhibited by tinfoil in layers forming steps. Other metals, namely platinum, lead, zinc and aluminum foil were similarly arranged and a table of the results recorded. § 63b.
THICKNESS. |
RELATIVE THICKNESS. |
DENSITY. |
|
|---|---|---|---|
| Platinum | .018 mm. | 1 | 21.5 |
| Lead | .050 mm. | 3 | 11.3 |
| Zinc | .100 mm. | 6 | 7.1 |
| Aluminum | 3.500 mm. | 200 | 2.6 |
He concluded from these data that the permeability increased much more rapidly than the thickness decreased.
84. Fluorescence and Chemical Action. § 70 and 63a.—Among the substances that fluoresced were barium platino cyanide, calcium sulphide, uranium glass, Iceland spar and rock salt. In producing sciagraphs on the photographic plates, he found it entirely unnecessary to remove the usual ebonite cover, which, although black, and so opaque to light, produced scarcely any resistance to the rays. The sensitive plate, even when protected in a box, could not be kept near a discharge tube, for he noticed that it became clouded. He was not sure whether the effect upon the sensitive plate was directly due to the X-rays or to a secondary action, namely, the fluorescent light which must have been produced upon the glass plate having the film, it being well known that light of fluorescence possesses chemical power. He called attention to the fact that inasmuch as fluorescent light which can be reflected, refracted, polarized, etc., was produced by the rays; therefore, all the X-rays which fell upon a body did not leave it as such. § 67. No effect was produced upon the retina of the eye although he temporarily concluded that the rays must have struck the retina in view of the great permeability of animal tissue and liquids. § 68, at end. Conclusions of this kind not based on experiment, are never reliable, even when offered by very high authorities. Again the rays were weak. Roentgen himself admitted that the salts of metals in solution (§ 82, near centre) rendered the latter rather opaque. The eye ball is continually moistened with the solution of common salt. Further than this, Mr. Pignolet noticed in Comptes Rendus, Feb. 24, ’96, an account of an experiment of Darien and de Rochas. In anatomy it is common to experiment on fresh pig’s eyes in order to make comparisons with human eyes. The above named Frenchmen submitted the former to X-rays. The eyes were but slightly permeable thereto.

The Physical Institute, University of Würzburg,
WHERE PROF. ROENTGEN HAS HIS RESIDENCE, DELIVERS HIS LECTURES, AND PERFORMS HIS EXPERIMENTS.
From photograph by G. Glock, Würzburg. (Not referred to in book.)
85. Non-refraction and But Little Reflection of X-rays.—He employed a very powerful refracting prism made of mica and containing carbon bi-sulphide and water. The same prism refracted light but did not refract X-rays. No one would think of making prisms for examining light, of ebonite or aluminum, but he made such a prism for testing X-rays. But if 74there were any refraction he concluded that the refractive index could not have been more than 1.05, which may be considered as a proof that the rays cannot be refracted. He tried heavier metals, but the difficulty of arriving at any satisfactory results was due to the resistance of such metals to the transmission of the rays. Among other tests was one consisting in passing the rays through layers of powdered materials through which the rays were transmitted in the same quantity as through the same substances not powdered. It is well known that light passed into powdered transparent materials, is enormously cut off, deviated, diffused, refracted etc., in view of the innumerable small surfaces of the particles. Hence he concluded that there was little if anything in the nature of refraction or reflection of X-rays. § 146. The powdered materials employed were rock salt, and fine electrolytic and zinc dust. The shadows, both on the screen and as recorded on the photographic plate were of substantially the same shade as given by the same materials of the same thickness in the coherent state. One of the most usual ways of testing refraction of light is by means of a lens. X-rays could not be brought to a focus with the lens of what ever material it was made. Among the substances tried were ebonite and glass. As expected, therefore, the sciagraph of a round rod was darker in the middle than at the edges; and a hollow cylinder filled with a more transparent liquid showed the centre portion brighter than its edges. If one considers this observation in connection with others, namely the transparency of powders, and the state of the surface not being effective in altering the passage of the X-rays through a body, it leads to the probable conclusion that regular reflection does not exist, but that bodies behave to the X-rays as turbid media to light, § 69.
86. Velocity of X-Rays In Different Bodies. p. 46.—Although he performed no direct experiment in this direction yet he inferred in view of the absence of refraction at the surfaces of different media, that the rays travel with equal velocities in all bodies.
87. Double Refraction and Polarization.—Neither could he detect any action upon the rays by way of refraction by Iceland spar at whatever angle the crystal was placed. As to this property of light see Huygen’s Works of 1690 and Malus’ Works of 1810. quartz also gave negative results. Prof. Mayer of Stevens Institute submitted to Sci., Mar. 27, ’96, the report of a crucial test for showing the non-polarization of X-rays. On six discs of glass, 0.15 mm. thick and 25 mm. in diameter, were placed very thin plates of Herapath’s iodo-sulphate of quinine. 75The axes of these crystals crossed one another at various angles. When the axes of two plates were crossed at right angles no light was transmitted; the overlapping surfaces of the plates appearing black. If the Roentgen rays be polarizable, the Herapath crystals, crossed at right angles, should act as lead and not allow any of the Roentgen rays to be transmitted. Prof. Mayer is well known as exceedingly expert in connection with minute measurements and in the manipulation of scientific experiments. Dr. Morton, Pres. Stevens Inst., attested the results as an absolute demonstration that X-rays are incapable of polarization. Stevens Indicator, Jan., ’96.
88. The Propagation of X-Rays Rectilinear.—There would be no difficulty in producing photographs of the bones of the hand with the rays of light, if it were not for the tremendous amount of reflection and refraction causing so much diffusion that no sharply defined shadow of the bones would be produced. By means of a powerful lens and a funnel pointed into a dark room, the author noticed that the condensed light thereby obtained when passed through the hand, and when the incident rays were parallel, came out so diffused that one would think that the light went through bones as easily as any part of the hand. An experiment of this kind serves to emphasize that the success of sciagraphy by X-rays is due not only to the great penetrating power, but to practically no refraction nor reflection. In view of the sharp shadows cast of objects even when located in vegetable or animal media, Roentgen was justified in giving the name of ray to the energy. He tested the sharpness of the shadow by making sciagraphs and fluorescent pictures not only of the bones of the hand, but of a wire wound upon a bobbin, of a set of weights in a box, of a compass, card and needle, conveniently closed in a metal case, and of the elements of a non-homogeneous metal. To prove the rectilinear propagation further, he received the image of the discharge tube upon a photographic plate by means of a pinhole camera. The picture was faint but unmistakable.
89. Interference. The rays of light may be caused to interfere with each other. See Newton’s Principia, Vol. III.; Young’s Works, Vol. I.—Theory points out that waves of ether of two pencils of light, when caused to be propagated at certain relative phases partially or wholly neutralize or strengthen each other. Roentgen could obtain no interference effects of the X-rays, but did not conclude that the interference property was absent. He was not satisfied with the intensity of the rays and therefore could not test the matter severely.

Fig. L.
90. Electrified Bodies Discharged by X-Rays. p. 47.—After Roentgen’s first announcement, others, and probably J. J. Thomson as the first, found that the X-rays would discharge both negatively and positively electrified bodies. Roentgen, in his second announcement, stated that he had already made such a discovery, but had not carried the investigation far enough to report satisfactorily on the details. At last he put forth an account of the whole phenomena and stated that the discharge varied somewhat with the intensity of the rays, which was tested in each instance by the relative luminosity of the fluorescent screen, and by the relative darkness produced upon the photographic plate in several instances. Electrified bodies, whether conductors or insulators, were discharged when placed in the path of the rays. All bodies whatsoever behaved in the same manner when charged. They were all discharged equally by the X-rays. He noticed that “If an electrical conductor is surrounded by a solid insulator such as paraffin instead of by air, the radiation acts as if the insulating envelope were swept by a flame connected to earth.” Upon surrounding said paraffin by a conductor connected to earth, the radiation no longer acted on the inner electrified conductor. The above observations led him to believe that the action was indirect and had something to do with the air through which the X-rays passed. In order to prove this, it was necessary for him to show that air ought to be able to discharge the bodies if first subjected to the rays, and then passed over the bodies. The apparatus for performing an experiment to test this prediction is shown in Fig. L, which serves to illustrate also the manner in which he prevented electro-static influences of the discharge tube, leading in wires and induction coil. § 71, near centre. For this purpose he built a large room in which the walls were of zinc covered with lead. The door for his entrance and exit was arranged to be closed in an air-tight manner. In the side wall opposite the door there was a slit 4 cm. wide, covered hermetically with a thin sheet of aluminum for the entrance of X-rays from the vacuum tube outside of the room. All the electrical apparatus connected with the generation of the X-rays was outside of the room. No force whatever came into the room, therefore, except the X-rays through the aluminum. § 71. In order to show that air which had been subjected to the X-rays would 77discharge a body immediately afterwards upon coming in contact therewith, he arranged matters so that the air was propelled by an aspirator. He passed air along a tube made of thick metal so that the rays could enter only through a small aluminum window near the open end. At over a distance of 20 cm. from the window was an insulated ball charged with electricity, and connected to any electroscope or electrometer. The professor used a Hankel electroscope. No published sketch was made by Roentgen; therefore, that shown in the figure was produced by inference from the description. The operation was as follows: The X-rays passed into the room through the aluminum window, and then into the metal tube through its aluminum window. When the air was at rest, the ball was not discharged. When the aspirator was at work, however, so that the air moved past the aluminum window and past the ball, the latter was discharged whether electrified positively or negatively. He modified the operation by maintaining the ball at a constant potential by means of accumulators, while the air which had been treated by X-rays was passed by the ball. “An electric current was started as if the ball had been connected with the wall of the tube by a bad conductor.” He was not sure whether the air would retain its power to discharge bodies as long as it remained out of contact with any bodies. He determined, however, that any slight “disturbance” of the air by a body having a large surface and not electrified, rendered the air inoperative. He illustrated this by saying that “If one pushes, for example, a sufficiently thick plug of cotton-wool so far into the tube that the air which has been traversed by the rays must stream through the cotton-wool before it reaches the ball, the charge of the ball remains unchanged when suction is commenced.” With the cotton-wool immediately in front of the window, it had no effect, showing, therefore, that dust particles in the air are not the cause of the communication of the force of the discharge from the X-rays to the electrified body. Very fine wire gauze in several thicknesses also prevented the air from discharging the body when placed between the aluminum window and the ball within the thick metal tube, as in the case of the cotton plug. Similar experiments were instituted with dry hydrogen instead of air, and, as far as he could discern, the bodies were equally well discharged, except possibly a little slower in hydrogen. He experienced difficulty in obtaining equally powerful X-rays at different times. All experimenters are acquainted with this difficulty. Further, he called attention also to the thin layer of air which clings to the surface of the bodies, 78and which, therefore, plays an appreciable part in connection with the discharge. § 16, near end. In order to test the matter further as to discharge of electrified bodies, he placed the same in a highly exhausted bulb and found that the discharge was in one case, for example, only 1/70 as rapid as in air and hydrogen at ordinary pressure, thereby serving as another proof that gas was the intermediate agency. Allowance should be made in all experiments in connection with the discharging quality of X-rays. The surrounding gas should be taken into account.
90a. Application of Principle of Discharge by X-Rays.—Professor Robb, of Trinity College, (Science, Apr. 10, ’96), proposed and explained and practically tested the principle of the discharge of X-rays to determine the relative transparencies of substances to X-rays. He plotted a curve in which the co-ordinate represented the charge of the condenser in micro-coulombs, and the abscissæ the time between charging and discharging the condenser. The same plan could be adopted, he suggested, for making quantitative measurements of the intensity of X-rays from different tubes or the same discharge tube at different times. J. J. Borgmann, of St. Petersburg, probably was the first to show that X-rays charged as well as discharged bodies. See The Elect., Lon., Feb. 14, ’96, p. 501. Soon, a similar announcement was made by Prof. Righi, of Bologna. § 90.
90A. Borgmann and Gerchun’s Experiments. Action of the X-Rays on Electro-static Charges and (La Distance Explosive.) Comptes Rendus, Feb. 17, ’96; from Trans., by Louis M. Pignolet.—A positively charged zinc disk connected to an electroscope lost its charge almost instantly and acquired a negative charge. When the charge on the zinc disk was negative, the loss was much slower and was not complete, a certain charge remaining. When the rays fell upon two small platinum balls connected to the terminals of an induction coil but separated beyond its sparking distance, sparking took place between them, showing that X-rays, like ultra-violet rays, increase the sparking distance of static charges.
90b. Righi’s Experiments. Bodies In The Neutral or Negative State, Positively Electrified By X-Rays. Comptes Rendus, Feb. 17, 1896. From Trans. by Louis M. Pignolet.—The measurements were made by this eminent Italian physicist, with a Mascart electrometer connected with the bodies upon which the X-rays impinged and enclosed in a grounded metallic case (Faraday cylinder) provided with an aluminum window for the entrance of the rays. A metallic disk connected with the electrometer lost its charge rapidly whether positive or negative.
79§ 99S. Initial positive charges were not completely dissipated; negative charges were not only completely dissipated but the bodies acquired positive charges. Disks in the neutral state were charged positively by the X-rays the same as takes place with ultra-violet rays. The final positive potential was greater for copper than for zinc and still greater for retort carbon (“le carbon de cornue”) 90c. at end. The various results are not conflicting if the particular materials are taken into accounts. 90c at end.
90c. The experiments of Prof. Minchin, an expert in such measurements, are properly described here, in that they seem to clear up the superficial ambiguity. He formulated the conclusion (The Elect., Lon., Mar. 27, ’96, p. 736) thus:—“The X-rays charge some bodies positively and some negatively, and whatever charge a body may receive by other means, the X-rays change it, both in magnitude and sign, to the charge which they independently give to the body.” Thus, in the case of magnesium, if the same is first positively charged by any suitable means, then will the X-rays not only discharge it, but electrify it negatively, while if this metal is first negatively charged, the X-rays either diminish or increase the discharge. It must be remembered, however, that this is not true with all metals, for he found that gold, silver, copper, platinum, iron, aluminum, bismuth, steel and antimony, are all positively electrified.
90d. Benoist & Hermuzescu’s Experiment. Negative Charges Dissipated Faster Than Positive By X-Rays. Rate Depends Upon Absorption. Law Formulated. Comptes Rendus, Feb. 3, Mar. 17 and April 27, ’96. They observed that the rays dissipated entirely the charge of electrified bodies in their path, and that negative charges were dissipated more rapidly than positive. § 99Q. They also noticed the discharge augments with the opaqueness of the body and that the effect is more considerable with two thin superposed sheets than with one. In experimenting upon the influence of the discharge of the gaseous dielectric in which the bodies were located, they formulated the following law. The rapidity of the dissipation of the electric charge of an electrified body under the same condition varies as the square root of the density of the gas surrounding the body. The dissipation of the electric charge depends upon the nature of the electrified body, due to a sort of absorbing power (§ 99M) connected with the opaqueness of the body and upon the nature of the surrounding gas, due to the density of the gas or when passing from one gas to another. (From trans. by Louis M. Pignolet.)
91. Before Roentgen published in his second paper of Mar. 809, ’96, an account of his focus tube, the Kings College published a description of an exactly similar one, represented in the cut. See Elec. Rev., Lon., Mar. 13. ’96, p. 340. The cathode is concave and the anode is formed of platinum and is plane and at such an angle that the X-rays generated, § 63b., on diffusion of internal cathode rays, will be thrown out through the thin walls of the bulb. § 55 and 57. As the rays emanate from a point, the shadows are much clearer, especially in conjunction with powerful rays permitting several feet between the object and the tube. Mr. Shallenberger was among the first, and was the first as far as the author knows (Elect. World, Mar. 7, ’96, see cut reproduced) to originate the use of an X-ray focus tube.
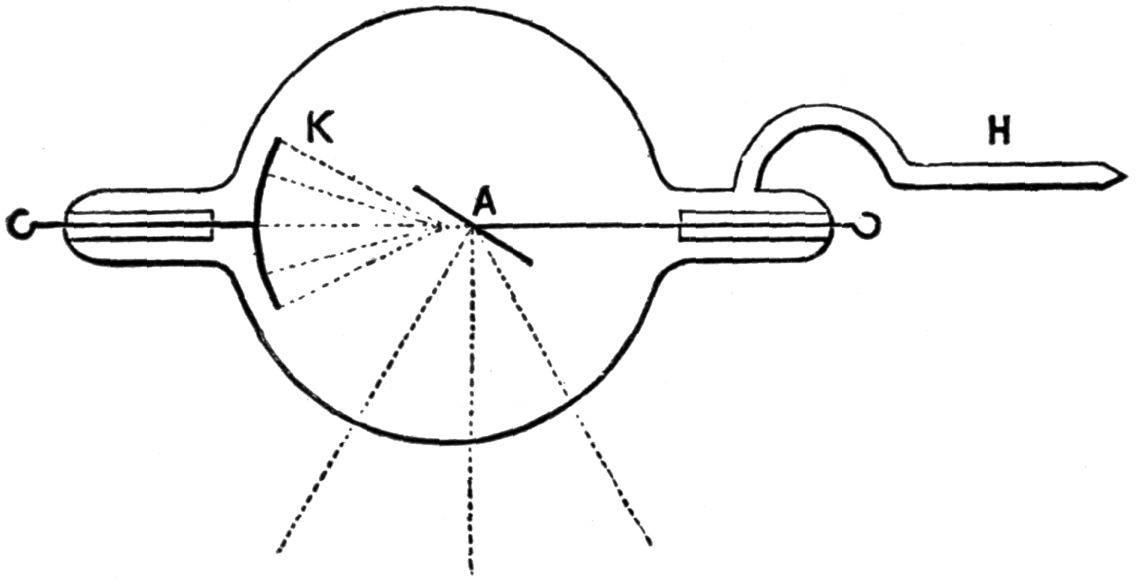
Typical Focus Tube.
91a. Apparatus Employed.—Prof. Roentgen paid tribute to Tesla, by alluding to the advantages resulting from the use of the Tesla condenser and transformer. In the first place, he noticed that the discharge apparatus became less hot, and that there was less probability of its being pierced. Again the vacuum lasted longer, at least in the case of his particular apparatus. Above all, stronger X-rays were produced. Again careful adjustment of the vacuum was not as necessary as with the Ruhmkorff coil.
92. X-Rays and Longitudinal Vibrations.—Prof. Roentgen did not consider X-rays and ultra-violet rays to be of the same nature, although they produced many common effects. The X-rays, as he found, by the above related experiments, behaved quite differently from the ultra-violet rays, which are highly refrangible, practically all subject to reflection, capable of being polarized, and absorbed according to the density of the absorbents. For valid reasons, the X-rays cannot be infra-red rays. While he does not affirm any theory, yet he suggests the theory of longitudinal waves for explaining the properties of X-rays. 81(This was not suggested again in his second announcement.) He stated that the hypothesis needs a more solid foundation before acceptance. The reason why Roentgen termed the energy X-rays is simply because X in algebra represents an unknown quantity.

Shallenberger Apparatus and Focus Tube. § 91.
93. At the Johns Hopkins University, U. S., in 1884, Sir William Thomson, (Kelvin) delivered a lecture in which he argued that the production of longitudinal vibrations, by electrical means, is reasonable and possible of occurrence. J. T. Bottomly, in Nature, Lon. Feb., (see also Elect. Eng., N.Y., Feb. 19, ’96, p. 187) called attention to this lecture as being of interest in view of Roentgen’s suggestion about longitudinal vibrations. Lord Kelvin called attention to what had been developed in connection with the electro-magnetic theory of light and referred to his own work in 1854, in connection with the propagation of electric impulses along an insulated wire surrounded by gutta percha, but he said that at that time no one knew the relation between electro-static and electro-magnetic units. The part of the lecture referring particularly to the possibility of longitudinal waves in luminiferous ether by electrical means reads as follows. “Suppose that we have at any place in air, or in luminiferous ether (I cannot now distinguish between the two ideas) a body that, through some action we need not describe, but which is conceivable, 82is alternately, positively and negatively electrified; may it not be that this will give rise to condensational waves? Suppose, for example, that we have two spherical conductors united by a fine wire, and that an alternating E. M. F. is produced in that fine wire, for instance, by an alternate current dynamo-electric machine, and suppose that sort of thing goes on away from all other disturbance—at a great distance up in the air, for example. The result of the action of the dynamo-electric machine will be that one conductor will be alternately, positively and negatively electrified, and the other conductor negatively and positively electrified. It is perfectly certain, if we turn the machine slowly, that in the air in the neighborhood of the conductors, we shall have alternately, positively and negatively directed electric force with reversals of, for example, two or three hundred per second of time, with a gradual transition from negative, through zero to positive, and so on; and the same thing all through space; and we can tell exactly what the potential and what the electric force are at each instant at any point. Now, does any one believe that, if that revolution were made fast enough, the electro-static law of force, pure and simple, would apply to the air at different distances from each globe? Every one believes that if the process can be conducted fast enough, several million times, or millions of millions times per second, we should have large deviations from the electro-static law in the distribution of electric force through the air in the neighborhood. It seems absolutely certain that such an action as that going on would give rise to electrical waves. Now, it does seem to me probable that these electrical waves are condensational waves in luminiferous ether; and probably it would be that the propagation of these waves would be enormously faster than the propagation of ordinary light waves.” Notice that the above was written twelve years prior to Roentgen’s discovery.
94. Prof. Schuster, in Nature, Lon., Jan. ’96, stated that the great argument against the supposition of waves of very small length lies in the absence of refraction, but questioned whether this objection is conclusive. He further stated: “The properties of the ether may remain unaltered within the greater part of the sphere of action of a molecule. The number of molecules lying within a wave length of ordinary light is not greater than the number of motes which lie within a sound wave, but, as far as I know, the velocity of sound is not materially affected by the presence of dust in the air. Hence there seems nothing impossible in the supposition that light waves, smaller than 83those we know of, may traverse solids with the same velocity as a vacuum. We know that absorption bands greatly affect the refractive index in neighboring regions; and as probably the whole question of refraction resolves itself into one of resonance effects, the rate of propagation of waves of very small lengths does not seem to me to be prejudged by our present knowledge. If Roentgen rays contain waves of very small length, the vibrations in the molecule which respond to them, would seem to be of a different order of magnitude from those so far known. Possibly, we have here the vibration of the electron with the molecule, instead of the molecule carrying with it that of the electron.”
95. Prof. J. J. Thomson showed how it was possible that “longitudinal waves can exist in a medium containing moving charged ions, and in any medium, provided the wave length is so small as to be compared with molecular dimensions, and provided the ether in the medium is in motion. It follows from the equation of the electro-magnetic field that the ether is set in motion in a varying electric field. These short waves would not be refracted, but in this respect they do not differ from transverse waves, which on the electro-magnetic theory would not be refracted if the wave length were comparable with molecular distances.” From Elect. Eng., N.Y., Mar. 18, ’96, p. 286, in reference to a paper before the Cam. Phil. So.
96. One of the very first questions asked in reference to a discovery is as to its practical utility. Already, we have important applications in one of the most humane directions, and that is in connection with diagnosis. Sciagraphs can also be employed in schools for the purpose of education, in some departments of anatomy, etc. The interest that it excites and the amusement that it affords are not to be overlooked, for anything in the nature of recreation possesses utility. However, we may greatly thank all experimenters who have investigated the subject, and who have not left its development alone to Roentgen; for predictions as to the utility of a discovery, however, apparently exaggerated, are very often proved, by subsequent developments, to have been underrated. Upon this point Prof. Boltzmann, in Zeit. Elect., Jan. 15, ’96, see also, The Elec., Lon., Jan. 31, ’96, p. 447, stated, “If we remember to what discoveries the most insignificant new natural phenomenon, such as the attraction of small objects by rubbed amber, of iron by the lode-stone, the convulsive twitches of a frog’s leg due to electric discharges, the influence of the electric current upon the magnetic needle, electro-magnetic induction etc., has led us, one can imagine to 84what applications an agent will be turned, which a few weeks after its discovery has given rise to such surprising results.”
97. Soon after hearing, (about the first of Feb. ’96,) of the Roentgen discovery, it occurred to the author to carry on experiments with fluorescence, but finding that it was inconvenient to work in a perfectly dark room, and, recognizing that black cardboard had practically no effect upon absorbing the X-rays, he devised a sciascope (daily papers, Feb. 13, and Elect. Eng., Feb. 19) which he afterwards learned was independently invented and used at about the same time by Prof. William F. Magie, of Princeton University, (see Amer. Jour. Med. Sci., Feb. 7, ’96 and Feb. 15, ’96) and by Prof. E. Salvioni, of Italy under the name of cryptoscope, (see Med. Sur. Acad. of Perugia, Italy, Feb. 8, ’96.) In about a month afterwards (Elect. Eng., N.Y., Apr. 1, ’96, p. 340) the instrument (with phosphorescent calcic tungstate § 13. in place of fluorescent barium platino cyanide) was again published under the name of the Edison fluoroscope. There are probably many other claimants—some professor in London—name forgotten. They all consist of a tapering tube with a sight hole at one end and a fluorescent screen in the other, which is closed by opaque card board. (Frontispiece at Chap. X). For the sake of conformity, the words sciagraph and sciagraphy and similar derivatives, and in view of the meaning of the radical definitions, have been employed throughout the book. The objection to the word fluoroscope is that the instrument is practically universally employed in seeing the shadows of objects, otherwise invisible to the naked eye, rather than to test fluorescence. The name sciascope was early suggested by Prof. Magie. For those who wish to make a screen, the author may state that he obtained a good one by mixing pulverized barium platino cyanide with varnish and spreading the mixture over a sheet of tracing cloth.
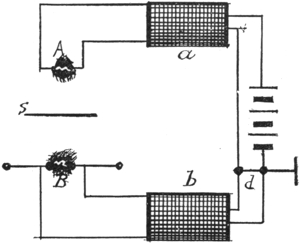
97a. Hertz’ Experiments. Electrified Bodies Discharged by Ultra-Violet Light of a Spark and by Other Sources of Light. Berlin Akad. II., p. 487, ’87. Wied Ann. XXXI, p. 983. English translation of the above. Lon. and N.Y. Macmillan, p. 63, ’93. From notes by Mr. N. D. C. Hodges.—This is the all-important initial work of H. Hertz. The source of light was a spark, and the great discovery resulted from a combination of circumstances and was unsought; but by studying and testing the matter, he found the cause. Two induction coils, a and b, having interrupter d, were included in the same circuit, as shown in the figure. The sparking of the active one (A) increased the length of the spark of the passive (B) § 10. He sought the cause. The discharge was more marked as the distance between the sparks was reduced. Sparks between the knobs had the same effect as those between points; but the effect was best displayed when the spark B was between knobs. The relation between the two sparks was reciprocal. The discharging effect of the active spark (A) spread out on all sides, according to the laws of light, first suggesting that light was the cause. Most solid bodies acted as screens, s. Liquid and gases served more or less as screens. The intensity of the action increased by the rarefaction of the air around the passive spark, i.e., in a discharge tube. The radiations from the spark, A, reflected from most surfaces, according to the laws of light, and refracted according to the same laws, caused the discharge. The ultra-violet light of the spark A was inferred to be the active agent in producing the discharge. The same effect was produced by other sources of light than the electric spark. The conclusions 86were afterwards confirmed by many, and subordinate discoveries originated. § 98—99T.
97b. Wiedemann and Ebert’s Experiment. Light Discharges Cathode, but Has No Influence upon anode, Nor Air-Gap. Different Gases and Different Pressures. Wied. Ann. XXXIII, p. 241. 1888. From notes by N. D. C. Hodges.—The arc light was used in place of the active spark of Hertz. Principal result was that the effect depended on the illumination of the cathode (§ 99.) The illumination of the anode or of the spark-gap did not influence the discharge. The very character of the charge was altered by the action of light upon the cathode. The influence of the illumination of the cathode did not consist solely at the starting of the spark, but lasted as long as the sparks continued to pass. With decreasing pressure of surrounding gas, the effect first increased (§ 97a) to a maximum, and then decreased (§ 54). The illumination had an effect on the path of the sparks, the path being perpendicular to the rays of light. The best results were obtained with carbonic acid gas. Hydrogen was next, and then air. They were contained in the tubes surrounding the poles. The character of the gas also had an influence on the rays which would produce the effect, with carbonic acid gas the effect showing itself even with the visible rays.
98. Elster and Geitel’s Experiment. Negatively Charged Bodies Discharged by Light. Wien. Berichte. Vol. CI, p. 703, ’92. Wied. Ann. Vols. XXXVIII, XXXIX, XLI, XLII, XLIII, XLIV, XLVI, XLVII, LII. Nature, Lon., Sept. 6, ’94, p. 451.—The elements employed for carrying on the experiment consisted of a delicate electroscope and certain metals, including aluminum, amalgamated zinc, magnesium, rubidium, potassium and sodium. Some of the experiments were made on the top of Mount Sonnblick, the same being 3,100 m. high, where the discharging power of light was found to be about twice as great as at Wolfenbuttel, which was at the level of 80 m. The whole time for the discharge was only a matter of a few seconds. The greater rapidity of discharge at the higher level was attributed to the greater proportion of ultra-violet rays (Hertz), which are the most easily absorbed by the atmosphere, according to Langley. All metals are not discharged alike by the action of light. The law follows the electro-positive series in such a way that the more electro-positive the metal, the longer the wave length of light necessary to produce the discharge. In experiments with potassium, sodium and rubidium, they made them successively, the cathode in a bulb of rarefied hydrogen. 87In this case it was found that the light of a candle, even at so great a distance as 7 m., would cause the discharge. Rubidium was sensitive in this respect to the red light from a heated rod of glass. Elster and Geitel were able also to discharge, by light, some non-metallic bodies, like calcic sulphide, when so prepared that it had the property of phosphorescing, and also darkly colored fluorites. Independently, the phenomenon is of importance, because Elster and Geitel determined that there was some common cause as to the discharge of bodies of light and the discharge from the earth’s surface. A series of experiments lasting three years, consisted in investigating the relation of the ultra-violet rays from the sun simultaneously to the quantity of charge in the atmosphere. The results acted as evidence of the explanation of the daily and annual variation of atmospheric potentials. These experiments are of importance in connection with X-rays, because Röntgen and Prof. J. J. Thomson subsequently, and possibly others independently, discovered that X-rays produce, not only a like, but a more extended action in that there is not so great a difference between their power to discharge negatively and positively electrified bodies. § 90a. In the further developments of their ideas, they tried the action of diffused day-light upon a Geissler tube traversed by vibrations which were produced by a Hertz vibrator (see recent book on Hertzian waves), the tube having an electrode of metal of the alkaline group. They were able to adjust the combination so that the presence of a little day-light would initiate a luminous discharge, while in the dark such a charge ceased. § 14a.
99. Elster and Geitel’s Experiment. Effect of Polarized Light upon the Cathode. Berlin Akad. ’95. Nature, Lon., March 28, ’95, p. 514. Proc. Brit. Asso., Aug. 16, ’94; Aug. 23, ’94, p. 406.—The X-rays have properties similar to those of light, and have their source in electricity. Quincke discovered that light which has been polarized perpendicularly to the plane of incidence is greatly increased as to its power of penetrating metals. Elster and Geitel used the following apparatus to determine the relation between polarized light and electricity. The current varied according to the angle of incidence and the plane of polarization. The apparatus comprised the following elements: An exhausted bulb, provided with a platinum anode, and a cathode consisting of potassium and sodium, combined in the form of a liquid alloy having a bright surface of reflection. The source of light was an oxyhydrogen flame, which played upon zircon instead of lime; a lens 88changed the diverging rays to parallel rays, which were polarized by a Nichol prism and allowed to fall upon the cathode. The electrodes of the vacuum bulb were connected to the poles of a generator of a current of about 400 volts. “The strength, of the current was greatest when the plane of polarization was perpendicular to the plane of incidence—i.e., when the electric displacements constituting light, took place in the plane of incidence, and when the angle of incidence was about 60°, i.e., the polarizing angle of the alloy itself.” Prof. Sylvanus P. Thompson confirmed these results by experiment. The rate of discharge was greatest, he said, when the plane of polarization was such that the Fresnellian vibration “chopped into” the surface. Polarized light, he reminded them, produced similar results upon selenium.
Although the domain of this book is necessarily limited to the consideration of phenomena connected with the internal and external energy of a discharge tube, yet if any other one subject is of special interest and utility in connection with the consideration of X-rays, it is that concerning the relation between the electric discharge and light, which has been thoroughly studied only during the past few years, and the accounts of the researches recorded in various periodicals and academy papers. Those readers, however, who desire to study this exceedingly interesting and novel branch of science, which in connection with the action of the internal cathode rays and X-rays upon electrified bodies, tends to uphold Maxwell’s theory as developed by mathematics and based upon early known facts and predicted discoveries, may find volumes upon this subject by referring to the citations below, named by Mr. N. D. C. Hodges and obtained by him by a search in the archives of the Astor Library. Of especial interest are those of Branly, § 99I, 99J, 99Q, 99S, 99T. Some notion as to the contents of the citations are given here and there.
99A. Koch’s Experiment. The Loss of Electricity From a Glowing Electrified Body. Wied. Ann., XXXIII., p. 454, ’88.
99B. Schuster and Anpenius’ Experiment. The Influence of Light on Electrostatically Charged Bodies. Proc. R. So., Lon., LXII., p. 371, ’87; Proc. Swedish Acad., LXIV., p. 405, ’87.—Many recent periodicals have set forth that ultra-violet light will discharge only negatively charged bodies. While this is practically or sometimes the case, yet these experimenters found that a positive charge was dissipated very slowly. They confirmed the results that the ultra-violet rays played the principle 89part in the removal of a negative charge. Polishing the surface accelerated the action. § 99, near beginning.
99C. Righi’s Experiment. Some New Electric Phenomena Produced by Light. Note 2-4, Rend. R. Acad. die Lincei, May 6, 20, and June 3, ’88.
99D. Righi’s Experiment. Some New Electric Phenomena Produced by Illumination. Rend. R. Acad. die Lincei. VI., p. 135, 187, ’88.—Confirmation of the results of other physicists, and a quantitative measurement determining that the E. M. F. between copper and selenium was increased 25 per cent. by illumination by an arc light. The selenium was in the form of crystals mounted upon a metal plate.
99E. Stolstow’s Experiment. Actino-Current Through Air. C. R., CVI., pp. 1593 to 95, ’88.—Liquids tested. Greatest absorbents of active rays most quickly discharged.
99F. Righi and Stolstow’s Experiments. Kind of Electric Current Produced by Ultra-Violet Rays. C. R., CVI, pp. 1149 to 52, ’88.—The discharge was accelerated by using a chemically clean surface. The burning of metals, for example, aluminum, zinc or lead in the arc light increased the discharging power.
99G. Bichat & Blondot’s Experiment. Action of Ultra-violet Rays on the Passage of electricity of Low Tension through Air. Comptes Rendus. CVI, pp. 1,349 to 51. ’88.—They employed arc lamps whose carbons had aluminum cores.
99H. Nacarri’s Experiment. The Dissipation of Electricity through the Action of Phosphorous and the Electric Spark. Atti di Torino. XXV, pp. 252 to 257. ’90.—The loss of charge was eighteen times less rapid in the dark through the air in a bottle, than when a piece of luminous phosphorous was placed in the bottle. The introduction of turpentine, which checked the glowing of the phosphorous, retarded the loss of charge.
99I. Branly’s Experiment. Photo-electric Current Between the two Plates of a Condenser. C. R. CX, pp. 898 to 901. ’91.—A positive charge was dissipated, and by a peculiar arrangement of the plates, screens, etc., and with particular materials, he was able to show that the rates of loss of a positive and negative charge were about equal. Numerous tests were instituted. If he is not mistaken, how closely related are X-rays and light. § 90. Those who wish to more thoroughly investigate this matter and verify the same, should study these experiments more in detail in connection with 91Schuster’s and Anpenius’ experiments (§ 99B), whose arrangement of the plates was the same as those of Branly.
99J. Branly’s Experiment. Loss of Both Electricities by Illumination with Rays of Great Refrangibility. C. R. CX, pp. 751 to 754. ’90.
99K. Righi’s Experiment. Electric Phenomena Produced by Illumination. Luer’s Rep. XXV, pp. 380 to 382. ’89.
99L. Borgmann. Actino-electric Phenomena. C. R. CVIII, p. 733. ’89. Jour. d. Russ. Phys. Chan. Ges. (2) XXI, pp. 23 to 26. ’89.—The photo-electric effect not instantaneous. A telephone served in the place of the galvanometer to detect the discharge.
99M. Stolstow’s Experiment. Actino-electric Investigations. Jour. d. Russ. Phys. Chan. Ges. (7-8) XXI, pp. 159 to 207.—It is necessary that the rays of light should be absorbed by the charged surface before having the discharging influence. § 99E. All metals are subject to the action, and also the aniline dyes. Two plates between which there is a contact difference of potential generate a current so long as the negative plate is illuminated. The effect is increased with the increase of temperature and is only found in gases, and is therefore of the nature of convection. He determined these principles by continuous work for two years. It should be remembered that in all these researches, the arc light is preferable, because the ultra-violet spectrum is six times as long as that given by the sun.
99N. Mebius’ Experiment. An Electric Spark and a Small Flame Employed. Bihang till K. Svenska Vet.-Akad. Hand. 15, Afd. 1, No. 4, p. 30, ’89.
99O. Worthington’s Experiment. Discharge of Electrification by Flames. Brit. Asso. Rep., ’90, p. 225.
99P. Fleming’s Experiment. Discharge Between Electrodes at Different Temperatures in Air and in High Vacua. § 99M, near end. Proc. Ro. So., LXVII., p. 118.
99Q. Branly’s Experiment. Hallwach and Stolstow’s Experiment. Loss of Electric Charge. Lum. Elect., LXI., pp. 143 to 144, ’91.—Branly obtained quantitative results. Hallwach found with the use of the arc light, a very small loss of positive electricity at high potentials; Stolstow, no such loss at potentials under 200 volts. Branly, with a 50 element battery and an arc light as the source of illumination, caused a discharge and thereby a constant deflection of 124 degrees of the galvanometer needle. The action of the light upon a positive disk caused a deflection of only three degrees by the same battery. 92With aluminum in the electrodes, the deflections were about 1400 and 24 respectively. Is it not sufficiently fully established that ultra-violet light will discharge not only negative but positive electricity? He experimented with substances heated to glowing or incandescence. Glass lamp chimneys at a dull, red heat, when covered with aluminum, oxide of bismuth, or lead oxides, withdraw positive charges. In the same way, for example, behaves a nickel tube in place of the lamp chimney.
99R. Wanka’s Experiment. A New Discharge Experiment. Abk. d. Deuts. Math. Ges. in Rrag., ’92, pp. 57 to 63.—He confirms the principle that the ultra-violet rays are the most powerful. A glass plate, which, as well known, cuts off most of the ultra-violet rays, was properly interposed and then removed and the difference noted.
99S. Branly’s Experiment. Discharge of both Positive and Negative Electricity by Ultra-Violet Rays. C. R., CXIV., pp. 68 to 70, ’92.—He further proves that ultra-violet rays of light will dissipate a positive charge. The experiments in this connection seem to prove more and more that the discharging power is only a matter of sufficiently high refrangibility of the rays of light.
99T. Branly’s Experiment. Loss of Electric Charge in Diffuse Light and in the Dark. C. R., CXVI., pp. 741 to 744. ’93.—A polished aluminum sheet was attached to the terminal of an electroscope properly surrounded by a metal screen. After a few days, the plate acted like any other metal plate polished or unpolished; it lost its charge very slowly, positive or negative alike, independently of the illumination. If it is then again polished, as for example, with emery paper and turpentine, it loses its charge rapidly in diffused light, which has passed through a pane of window glass, for example. Therefore, the ultra-violet rays are not alone effective, although most effective. The longer the time elapsing, after polishing, the slower the discharge takes place. Zinc behaved likewise, only more slowly. Other metals were tried. Bismuth acted differently from most metals. Whether charged positively or negatively, they exhibited rapid loss in the dark, in dry air under a metal bell, independently of the state of the polish.
100. Thomson’s Experiments. Elect. Eng., N.Y., Mar. 11, Apr. 8 and Apr. 22, ’96. Elect. Rev., N.Y., Apr. 8, ’96, p. 183. Stereoscopic Sciagraphs. Elect. World, N.Y., Mar. 14, ’96.—Prof. Elihu Thomson, of the Thomson-Houston Electric Company, described experiments to determine the practicability of making stereoscopic pictures by X-rays. A solid object may be considered as composed of points which are at different distances from the eye. By monocular vision, the solidity of an object is not assured. However, by the use of both eyes, the objects appear less flat. The experimenter used, as the different objects, a mouse, also metal wires twisted together, and, again, a block of wood having projecting nails. In order to produce a stereoscopic picture with X-rays, he took a sciagraph in the ordinary way. He then caused the relative displacement of the discharge-tube and the object, and took another sciagraph. By mounting the two sciagraphs in a stereoscope, he found that the effect was as expected, and in the case especially of the skeleton of the mouse, it was very curious,—less like a shadow picture and more like the real object. The picture was more realistic, as in the well-known stereoscope for viewing photographs.
101. Thomson’s Experiment. Manifolding by X-rays.—If one desires to take a print of a negative, for example by means of sun-light, it is evident that, on account of the opacity of the photographic paper, only one sheet would be placed under the negative for receiving a print. However, the X-rays are so penetrating in their power that it is possible for them to produce sciagraphs through several sheets, and thereby to result in the production of several pictures of the same object with one exposure. Without an experiment to prove this, one might argue that the chemical action of one sheet would absorb all the energy. The experiment of Prof. Thomson shows that this is not so. The elements were arranged as follows: First a discharge tube; then an object, namely, a key escutcheon of iron; then yellow paper; then paste board; then black paper; 95then two layers of albumen or sensitized paper; then two célérité printing papers; then two platinum printing papers; then one célérité; then six layers of sensitive bromide paper; then four layers of heavy sensitive bromide paper (heavier); then three layers of black paper, and finally, at the maximum distance from the discharge-tube, a sensitive glass plate of dry gelatine, with its face up, thereby making twenty-five layers in the aggregate. It is interesting to notice that an induction coil was not employed, but a small Wimshurst machine, having connected to each pole a small Leyden jar. § 106. 1,200 discharges occurred during exposure. The results were as follows: No sciagraphs developed upon the albumen, célérité nor platinum, which, it should be noticed, were merely printing papers. § 128. The impressions on the ten bromide papers were weak. See Multiple Sciagraphs, Fig. 2, p. 94. He attributed the reason of this to the thinness of the film. Although the glass plate was furthest away from the discharge tube, yet the impression was greater than on any of the papers, the result being shown in Multiple Sciagraphs, Fig. 1, p. 94. He suggested that the plates for use with X-rays should have unusually thick films. Incidentally he found that the intensifying process could be employed with profit to bring out the small details distinctly. Dr. Lodge also recommended thick films. See The Elect., Lon., Apr. 24, ’96., p. 865.
101a. Lumière’s Experiment. Enormous Transparency of Sensitive Photographic Paper. Comptes Rendus, Feb. 17, ’96. Translated by Mr. Louis M. Pignolet.—With a ten-minutes exposure, objects were sciagraphed through 250 super-imposed sheets of gelatino-bromide of silver paper, to observe the absorption of the X-rays by the sensitive films. The one hundred and fiftieth sheet was found to have an impression.
102. Proposed Double Cathode Tube. See also Elect. Rev., N.Y., Apr. 15, p. 191.—The nature of this will be apparent immediately from the cut which is herewith presented and entitled “Standard X-Ray Tube.” With unidirectional currents the concave electrodes in the opposite ends may each be a permanent cathode, while the upper terminal connected to the angular sheet of platinum may be the anode. Cathode rays, therefore, will be sent out from each concave disk, and striking upon the platinum will be converted into X-rays, assuming that the platinum is the surface upon which the transformation from one kind of ray to another takes place. § 63, at end. This is called a standard tube, because it may be employed with efficiency with any kind of generator. § 8a, 26a, 115, 116 and 145. It is interesting 96to notice a confirmation of the efficiency of such a tube, for Mr. Swinton, in a communication to the Wurz Phys. Med. So. (see The Elect., Lon., and Elect. Eng., N.Y., June 3,) showed and described a picture of an exactly similar tube. By an experiment, the tube operated as expected. First proposed by Prof. Elihu Thomson, who is an author also of the following experiment:
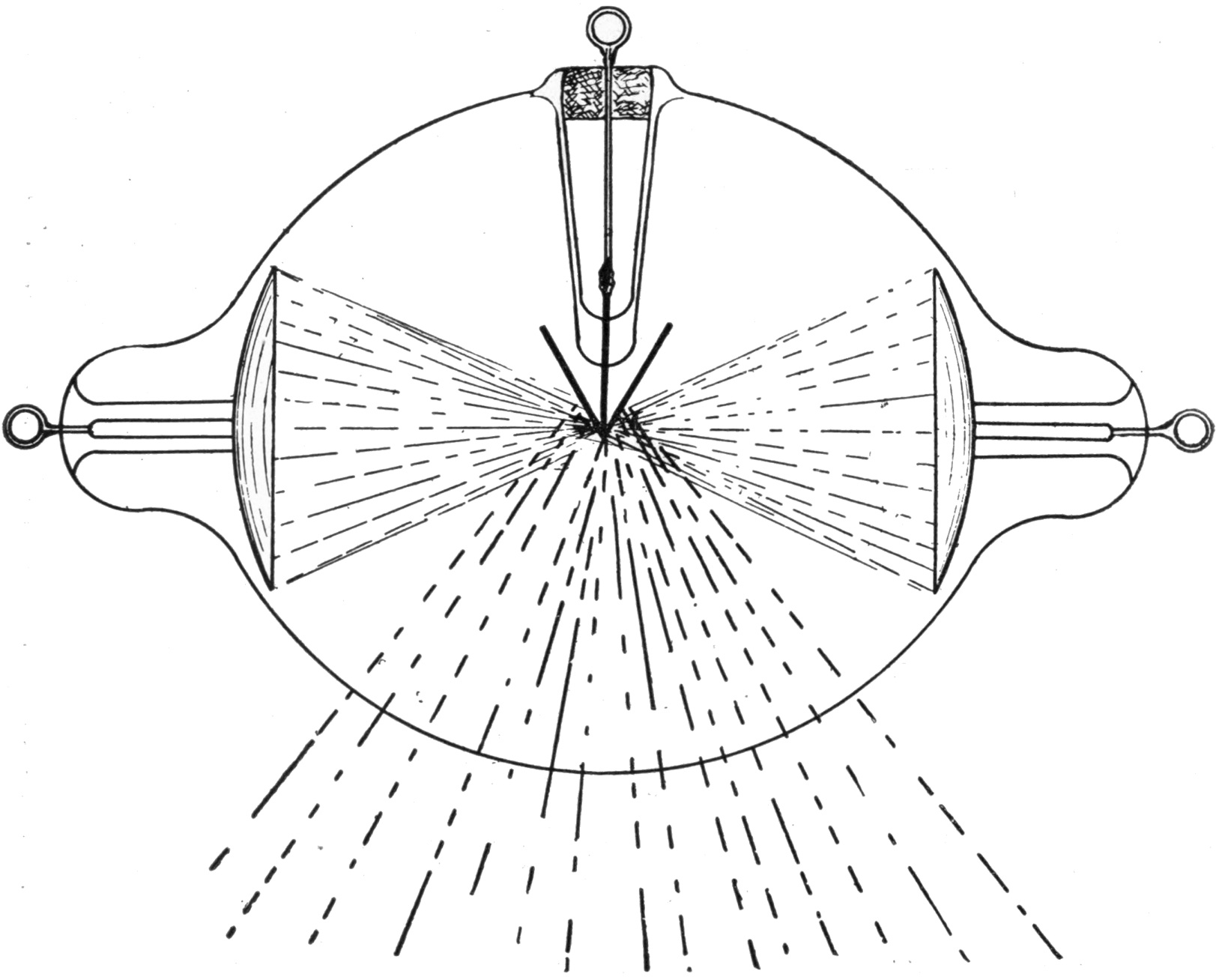
Standard X-Ray Tube.
103. X-Rays. Opalescence and Diffusion. Elect. World, Apr. 25, ’96.—He alluded to opal glass and milk to illustrate that light is reflected not only at the surface of a body, but from points, or molecules, or particles, located underneath the surface. By some experiments with X-rays, he found that they had a similar property only not to such a large per cent., but on the other hand by the way of contrast, there are many more substances opalescent to X-rays than there are to light, for the reason that the former will penetrate more substances and to greater distances. He made many observations with a modified sciascope, § 105, by pointing it away from the discharge tube and towards different substances struck by X-rays. To all appearances, such substances became the sources of the X-rays. He alluded to Mr. Tesla’s experiments on reflection, § 146, but 97noticed that there was a slight difference between reflection and diffusion and he was satisfied that reflection took place from the interior of the substances as well as from the surface. Metal plates, he said, gave apparently little diffusive effect, appearing to reflect feebly at angles equal to the incident angles. He alluded to Edison’s experiment also, § 133, with a large thick plate cutting off the X-rays and attributed the luminosity of his modified sciascope to rays both reflected and diffused from surrounding objects, which generally as a matter of course, are more of non-metallic objects than metallic, such as floor, ceiling, walls, tables, chairs and so on. Evidently, the interior of one’s hand causes diffusion; very little, however, for a sciagraph by means of a focus tube gives wonderfully clear outlines, and yet the rays do not come from a mathematical point. § 88. Prof. Thomson acknowledged that independently of himself, Dr. M. I. Pupin, of Columbia College, had reported in Science, Apr. 10, ’96, see also Electricity, Apr. 15, ’96, p. 208, upon investigations on the same general subject, namely diffusion, and also referred to experiments of Lenard, § 69, and Roentgen on diffusion. Agrees also with experiments of A. Imbert and H. Bertin-Sans in Comptes Rendus, Mar. 2, ’96. He suggested that this property of diffusion acted as an explanation why sciagraphs can never have absolutely clearly cut shadows of the bones or other objects imbedded in a considerable depth of flesh.
103a. A. Imbert and H. Bertin-Sans’ Diffusion and Reflection in Relation to Polish. X-Rays. Comptes Rendus, Mar. 2, ’96. Translated by Louis M. Pignolet.—They concluded, under the conditions of their experiments, that if X-rays were capable of reflection it was only in a very small proportion; on the other hand, the rays can be diffused en assez grande quantité, the intensity of the diffusion appearing to depend much more upon the nature of the diffusing body than upon its degree of polish. From this they attributed to the rays a very small wave length, such that it would be impossible to get in the degree of polish necessary to obtain their regular deflection. Perrin attempted unsuccessfully to reflect the rays from a polished steel mirror and a plate of “flint,” but with exposures of one hour and seven hours respectively, nothing was obtained. From trans. by L. M. Pignolet, Comptes Rendus, Jan., 96. By exposing a metal plate to the rays and suitably inclining it in front of the opening, Lafay also proved reflection, for it was possible to discharge the electrified screen; hence, as he called it, diffused reflection. Comptes Rendus, Apr. 27, ’96; from trans. by L. M. Pignolet.
98104. Fluorometer.—He constructed an instrument for comparing the merits of different discharge tubes, and for indicating the comparative luminosity of different screens subjected to the action of the same discharge tube. The instrument served also to act as an indicator of the diffusing power of different materials. “By placing two exactly similar fluorescent screens at opposite ends of a dark tube, and employing a Bunsen photometer screen, movable as usual between the screens, a comparison of the diffusing power of different materials might be made by subjecting the pieces placed near the ends of the photometer tube outside, to equal radiation from the Crookes’ tube.” From Prof. Thomson’s description.
The author performed some experiments (Elect. Eng., N.Y., Apr. 15, ’96, p. 379) in relation to candle-power of X-rays by looking into a sciascope and moving it away until the luminosity just disappeared. He then detached the black paper cover from the phosphorescent screen and pointed the sciascope toward a candle flame and receded away until the fluorescence also disappeared. The distances, with different candles, would, of course, somewhat vary, but it would in the rough be a constant quantity, while different discharge tubes would cause the vanishing fluorescence at different distances. Now, assuming that the X-rays vary inversely as the square of the distance, as believed by Röntgen, their power to fluoresce could, therefore, always be named as so much of a candle-power.
105. Simple Device for Comparing and Locating the Source and Direction of X-rays. Phosphorescence Not Essential.—In the ordinary sciascope, the fluorescent screen is located at one end, and the eyehole at the other. He modified this construction by employing a long straight tube, made of thick metal, so that X-rays could not enter through the wall. About at the centre of the tube was a diaphragm of a fluorescent material. Now, it is evident that if this is directed toward the phosphorescent spot and placed very close to the same, and the other end be looked into, the screen will become fluorescent, if X-rays are emitted from the area expected. Such a result occurred. With this instrument, he was able to show, in a similar way, that X-rays did not come from the anode, nor from the cathode directly. In one case, he provided a piece of platinum within the discharge tube, in such a position as to be struck by the cathode rays. § § 91 and 116. The instrument showed that X-rays radiated from the platinum, although the latter was not luminous nor phosphorescent,—illustrating again that phosphorescence is not a necessary accompaniment of X-rays, and assisting 99in upholding the principle that as the phosphorescence diminishes by increase of vacuum and increase of E. M. F., the X-rays increase. It should be noticed that Prof. Thomson emphasizes that the tube should be made of thick metal.
106. Rice’s Experiment. Apparatus for Obtaining X-Rays. § 109, 114, 131, 137. Tube Energized by a Wimshurst Machine. Elect. Eng., N.Y., Apr. 22, p. 410.—Roentgen had always employed the induction coil. As to those who first excited the discharge tube by the Holtz or Wimshurst machine or generators of like nature, it is not certain; but, according to public records, they were independently Prof. M. I. Pupin, of Columbia College, and Dr. William J. Morton, of New York. See Electricity, N.Y., Feb. 19, ’96. The accompanying cut marked “Rice’s Experiment, Fig. 1,” is a diagram representing the several elements of the apparatus, while “Rice’s Experiment, Fig. 2,” shows what kind of a sciagraph can be obtained by means of a Wimshurst machine. § 101, at centre. The details of the apparatus as employed by Mr. E. Wilbur Rice, Jr., Technical Director of the General Electric Co., were as follows: A Wimshurst machine, having a glass plate 16 inches diameter, coupled up with the usual small Leyden jars, spark under best conditions of atmosphere, etc., 4 inches. “The usual method of taking pictures with such a machine is to connect the interior coatings of the two jars, respectively, to the positive and negative conductors of the machine, the terminals of the discharge tube being connected between the external coatings of the Leyden jars. In this condition, the disruptive discharge of the Leyden jars passes through the tube and across the balls upon the terminals of the conductors of the machine, the length of spark being regulated by separating the balls in the usual way.” Later, he found that by omitting the Leyden jars, the generation of the X-rays was practically non-intermittent. He therefore connected the terminals of the discharge tube directly to those of the Wimshurst machine as indicated in “Rice’s Experiment, Fig. 1,” which also illustrates the details in the carrying out of the experiment for obtaining the picture, Fig. 2, of the purse containing the coins and a key. The principal feature was the introduction of a lead diaphragm containing a small central opening 7-8 inch diameter opposite the fluorescent spot. Sciagraphs taken thus required a little more time, about 60 minutes, while without the diaphragm, the time could be shortened to about 30 minutes, but the shadows were not so clear in the latter case. The figures are on p. 100.
101107. Source of X-Rays Tested by Propagation Through a Small Hole.—This would illustrate not only that the fluorescent spot is the source of X-rays, but also that a very small portion comes from other parts that are probably bombarded by stray cathode rays (due to irregular surface of cathode § 57, or by reflected X-rays or cathode rays.
He tested the source of the X-rays by means of the following arrangement of the apparatus: It will be noticed that the lead diaphragm is quite close to the fluorescent spot. Upon holding the sciascope on the opposite side, and pointing it toward the spot, the luminous area of the fluorescent screen was about the same as that of the opening in the diaphragm, but the size grew rapidly upon receding from the diaphragm. If the rays had come from the cathode, however, the fluorescent spot on the screen would not have increased in size so rapidly during recession, and, therefore, the rays must have come from the spot on the glass struck by the cathode rays. § 113, 116, 117.
107a. Leeds’ and Stokes’ Experiment. Use of Stops in Sciagraphy. Western Electrician, Mar. 14, ’96.—In order to obtain clear definitions of the shadows, Messrs. M. E. Leeds and J. B. Stokes provided lead plates with holes, varying in size from 1/4 inch to an inch between the discharge tube on one side and the object and photographic plate on the other. In this manner they obtained excellent sciagraphs of animals having very fine skeletons. See the picture of the rattlesnake at § 135 and of a fish on page 63. See also the frog taken abroad page 90.
107b. Macfarlane, Morton, Klink, Webb and Clark’s Experiment. X-Rays From Two Phosphorescent Spots. Elect. World, Mar. 14, ’96.—By means of nails projecting vertically from a board (similar to the process carried out by Dr. William J. Morton, Elect. Eng., N.Y., Mar. 5, ’96), they proved, undoubtedly, that the source of the X-rays was at the surface of the glass directly opposite the cathode. By modification, which acted as further proof, a tube was provided with a cathode at the centre. There was a phosphorescent spot at each end. One board was placed laterally to the tube, and two shadows of each of certain nails were cast, which were caused as proved by measurement, by a double source of X-rays. This experiment illustrates the importance of preventing double shadows. The plate should be perpendicular to the line joining the two sources of the X-rays when there are two such sources. Even with the focus tube Dr. Philip M. Jones, of San Francisco, determined that there were two phosphorescent spots. These should be taken into account in all cases and attempts made to 102produce but one strong focus upon the platinum. Elect. World, N.Y., May 23, ’96.
104108. Stine’s Experiments. Source of X-rays Determined by Sciagraphs of Short Tubes. Elect. World, N.Y., Apr. 11, ’96, pp. 392, 393.—Prof. Stine, of the Armour. Inst. of Tech., by means of the diagram shown in Fig. 1, p. 102, clearly proved that the X-rays have their source at the area struck by the cathode rays located directly opposite the disk marked “cathode.” If the reader will investigate the diagram and the sciagraphs, he will obtain a clearer knowledge of the evidence than by any verbal description, further than to explain how the elements are related to one another. In Fig. 1, therefore, will be noticed covered photographic plates, located as indicated with reference to the extreme left-hand end of the discharge tube, where the cathode rays strike. The surface of Plate 5 is parallel to that of the cathode, and the phosphorescent spot is in line between the two above named elements. The result is shown in Fig. 2, p. 102, the objects sciagraphed being several short sections of tubes with diameters varying from 1/2 to 3 inches.
A, in Figs. 3, 4, p. 104 and in Figs. 5, 6, p. 112, identifies the ends lettered A in Fig. 1. The sciagraph in Fig. 3 was obtained on the plate shown at the top in Fig. 1; that in Fig. 4, on Plate 2; that in Fig. 5, on Plate 3; and that in Fig. 6, on Plate 4. Not only were direct shadows visible, but also secondary shadows, indicating, therefore, that, although the source of practically all the rays was at the phosphorescent spot, yet a portion of the rays came slightly from other directions, either by reflection or by actual production of rays, upon other portions of the tube. Look now especially at Fig. 3, p. 104. If the rays came from the anode, then would this appearance necessarily be the same as that in Fig. 2. Similarly, the other sciagraphs may be considered to show that the rays do not come from the anode. In the case of the sciagraphs in Figs. 4, 5 and 6, only a single tube acted as the body for casting a shadow. Prof. Stine stated that the experiments were repeated over and over again, thereby establishing the phenomena as uniform.
109. Stine’s Electrical Apparatus Employed. § § 106, 112, 114, 131, 137.—Prof. Stine gave the following suggestive points:
“Among the first points investigated was the influence of the interrupter. The coil was provided, first with the familiar mercury make and break, and then an ordinary vibrator. The mercurial device gave very good results.
The small interrupter was found the more reliable, and seemed to shorten the needed time of exposure. A rotary contact-maker, 105giving two interruptions of the current per revolution, was also tested. This was driven by a motor with a condenser capacity of fourteen microfarads connected across the brushes. Owing to the large capacity of the condenser, a heavy current could be broken without marked sparking. The circuit breaker was tested at speeds ranging from 500 to 4,000 per minute, to note the influence on the time of exposure. The best results were obtained at the lower speed.... As no especial advantage could be noted when using the mercury breaker, it was abandoned for the vibrating interrupter.” This point is noted in detail, since so many experimenters seem to prefer such cumbersome devices, but they are, in reality, unnecessary.
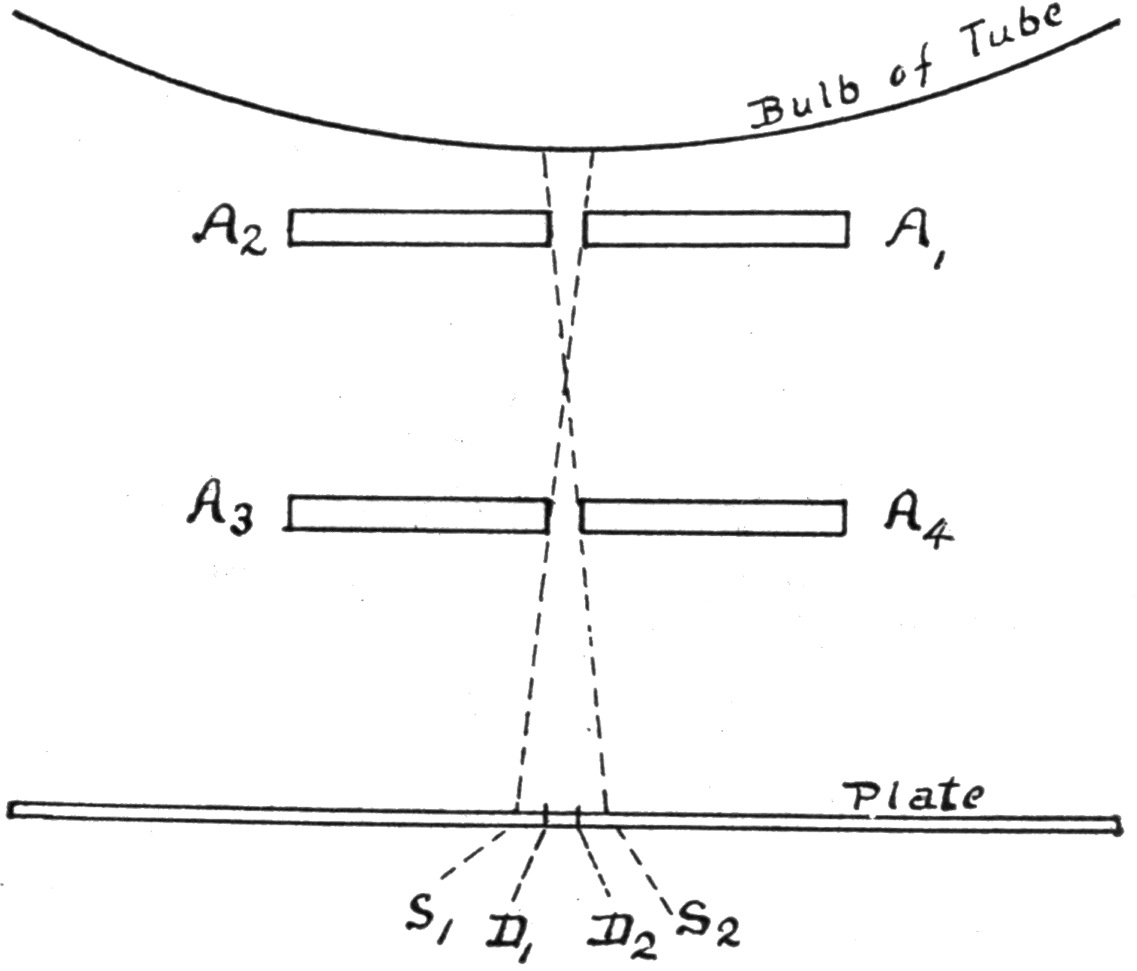
Stine’s Experiment, Fig. A. § 110.
110. Apparent Diffraction of X-Rays Really Due to Penumbral Shadows. Elec. Eng., Apr. 22, ’96, p. 408.—By referring to the diagram marked “Stine’s Experiment, Fig. A,” the arrangement of the elements may be seen, while the photographic print is shown in “Stine’s Experiment, Fig. B.” p. 106. Prof. Stine described the investigation as follows: Diffraction is naturally one of the first kinematical points to be investigated in the Roentgen experiments. It was noticed that when the opaque object was some distance from the plate, pronounced penumbral shadows resulted. These were of such width as to indicate diffraction. However, when such shadows are plotted back to the tube they are found to be purely penumbral, and not 106caused by diffraction. To completely demonstrate this point the experiment illustrated in Fig. A was undertaken. Here A1 to A4 are brass plates one inch wide and 1/8 inch thick, and of the length of the dry plate employed. They were first fastened together, so as to leave two parallel slots 1/8 of an inch wide. These plates are placed within 3/8 of an inch of the bulb, were one inch apart, and rested 1-1/8 inches above the dry plate. The resulting sciagraph is shown in Fig. B. In the diagram S1 S2, the edges of the penumbral shadow are very sharp and distinct. The direction of the rays is indicated, showing that there was absolutely no diffraction. This experiment has been modified in a variety of tests, with always the same result.”
110a. Jean Perrin’s Non-Diffraction. Comptes Rendus, Jan. 27, ’96. From trans. by Louis M. Pignolet.—The active part of a tube was placed before a very narrow slit; 5 cm. further, there was a slit 1 mm. wide; 10 cm. further, there was the photographic plate. An exposure of nine hours gave an image with sharply defined borders, upon which there was no diffraction fringe.
Stine’s Experiment. Fig. B. § 110.
159a. Non-Refraction.—Refraction was attempted with prisms of paraffine and of wax, but no refraction was noticed.
111. Scribner and M’Berty’s Experiment. Source of X-Rays Determined by Interception of Assumed Rectilinear Rays From the Cathode. Elect. Eng., N.Y., Apr. 8, ’96, p. 358; Amer. Inst. Elec. Eng., Mar. 25, ’96. West. Branch.—Refer now solely to Fig. 1, S. and M.’s experiment. Notice the relative arrangement of the elements. First, the discharge tube with the cathode at the upper part and the phosphorescent spot opposite thereto; then below a thick lead plate with a single opening; then a second lead plate with two small openings placed laterally at such a distance that if there were rectilinear rays from the cathode they could not strike (by passing through the small hole), the covered photographic plate which was the next element in order. The description did not state that the photographic plate was covered, but the experimenters must have had the usual opaque cover upon it or else the luminous 107rays could have produced images. The developed plate showed two spots strongly acted upon and surrounded by portions which were less acted upon, the same as would be produced by light radiating from a surface as distinguished from a point. From the fact that they stated that the exposures were very long, it may be concluded also that the plates were covered by a material opaque to ordinary light. Measurement showed that the rays which produced the images came from the phosphorescent spot (§ 106, 109, 114, 131, 139) and not from the cathode directly by rectilinear propagation.

S. & M.’s Experiment, Fig. 1. & 2.
112. Source on inner Surface of the Discharge Tube Determined by Pin-Hole Images. Reference may now be made to S. and M.’s Experiment, Fig. 2.—The discharge tube has, as before, a cathode on one side, and the phosphorescent spot during operation on the opposite side. Lead plates were provided in positions indicated by the heavy black straight lines, there being a pin hole in each one. Behind these lead plates, measured from the discharge tube, were the covered photographic plates, as indicated. By measurement, it was afterwards determined that practically all the X-rays started from the phosphorescent spot. The electrode was put in an oblique position, as indicated, so that the same would not obstruct any X-rays trying to pass through the pin hole in the uppermost plate. The experiment served specifically to show that the X-rays started from the inner surface of the glass, because images produced on the upper and lower plates were equally strong. Perrin also found that the X-rays are developed at the interior sides of the tubes. (Comptes Rendus, Mar. 23, ’96. From trans. by L. M. P.) The rays, in producing each image, had to pass through an equal thickness of glass. If the rays had come from the outer surface, for example, two thicknesses would have been traversed by the rays striking the upper plate, and no thickness by those impinging upon the lower plate. That no rays came from any 108other portion or element of the discharge tube was evident, because a picture of the phosphorescent spot was the only one produced, and this picture was inverted, as usual, with pin hole cameras. (A pin-hole camera is the same as any other, with the lens replaced by a very small hole, which acts as a lens.)
In the way of further evidence, if not enough already, Meslans early determined that the phosphorescent spot on the glass is the source of X-rays (Comptes Rendus, Feb. 24, ’96. From Trans. by Mr. Louis M. Pignolet).
Jean Perrin’s Experiments. The Origin of X-rays. Comptes Rendus, Mar 23, ’96. From Trans. by Louis M. Pignolet.—He also confirmed that X-rays radiate from the phosphorescent spot.
112a. De Heen’s Experiment. The Anode Believed to be the Source of X-rays. Comptes Rendus, Feb. 17, ’96. From trans. by Louis M. Pignolet.—A lead screen, pierced by several holes, was placed between the discharge tube and the photographic plate. The shadows of the holes on the plate indicated that the rays emanate from the positive pole of the tube.
As both Thomson (E.) and Rowland, as well as De Heen, at first concluded likewise, is it not probable that the anode was struck by the cathode rays (see § § 113, 116)? For it was fully admitted that the anode, otherwise, does not emit X-rays.

113. Lodge’s Experiment. X-rays Most Powerful when the Anode is the Part Struck by the Cathode Rays. Pin-Hole Pictures by X-rays to Determine Source of X-rays, and Pin-Hole Images upon Glass Compared. The Elect., Lon. Apr. 10, ’96, p. 784.—The object of the experiment was to confirm, if possible, by a modified construction, the source of the X-rays, as being the surface struck by cathode rays, whether the surface is that of glass or any other substance. He had constructed, for this purpose, a discharge tube, as illustrated in the diagram, which may be seen, at a glance, to contain a concave electrode at one end, and a flat electrode at the other. Between them, and connected to the concave electrode, is an inclined sheet of aluminum, shading both electrodes. The wires leading to the aluminum sheet are well protected by glass. He arranged matters so that either the concave or the flat electrode could be made positive or negative. The operation consisted first in taking through a pin hole, 1/4 of an inch in diameter, X-ray pictures on photographic plates, from different 109points, at measured distances. After these were taken, glass plates received the luminous images at the positions of the sensitive plate. Pencil drawings were then made, and compared with the X-ray pictures. The experiment involved also the repetition of this operation, except that the polarity of the terminals was changed.
“When the small flat disk was cathode, every part of the complicated anode appeared strongly and quickly on the plate, especially the tilted and first bombarded portion on a photographic plate placed above the tube. The cathode disk itself did not show at all. On a plate placed below the bulb, the anode cup appeared strong, but the tilted disk did not appear. On the other hand, … its focus spot acted as a feeble point source, by reason of a few rays reflected back on to it from the cup.
“When the current was reversed, the small disk anode showed faintly, being excited by rays which had penetrated the interposed tilted disk, but again the cathode hardly showed at all, not even the tilted portion on a plate placed below the bulb. This is confirmed by J. Perrin. In no case could an image of the cathode be obtained. (Comptes Rendus) Mar. 23, ’96. From trans. by L. M. P.) By giving a very long exposure (two hours), some impression was obtained by Dr. Lodge about equal to that from the shaded anode disk; but, of course, if the tilted plate had been under these circumstances an anode, it is well known that a few minutes would have sufficed to show it strong upon the plate beneath.
“Hence, undoubtedly, the X-rays do not start from the cathode or from anything attached to the cathode but do start from a surface upon which the cathode rays strike, whether it be an actual anode or only an ‘anti-cathodic’ surface. Best, however, if it be an actual anode. (Independently discovered by Rowland, § 116. and Roentgen, § 91.”)
“When the glass walls, instead of receiving cathode rays, are pierced only by the true Roentgen rays from the disk in the middle, no evidence is afforded by my photograph that the glass under these circumstances acts as a source. It is well that it does not, for its only effect would be a blurring one. § 91. With focus tubes, the glass phosphoresces under the action of the X-rays as anything else would phosphoresce, but its phosphorescence is not of the least use. It is a sign that a tube is working well, and that the rays are powerful; but if by reason of fatigue (§ 58) the glass ceases to phosphoresce strongly, the fact constitutes not the slightest detriment.”
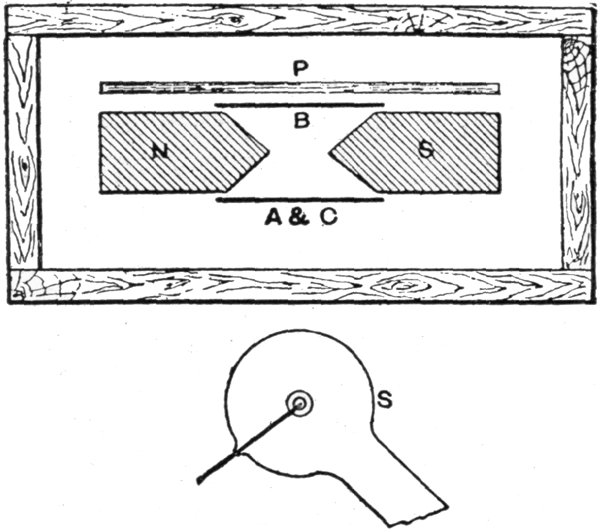
X-Ray Uninfluenced by a Magnet. Severe Test.—His first experiment on magnetic deflection, the sciagraph of a magnet with a background of wire gauze, only showed that if there were any shift by reason of passage of rays between the poles it was very small; but he definitely asserted, as in accompanying diagram, that a further experiment has been made which effectually removes the idea of deflectibility from his mind, and confirms the statement of Professor Roentgen. § 79. A strong though small electro-magnet, with concentrated field, had a photograph of its pole-pieces taken with a couple of wires, A and C, stretched across them on the further side from the plate—nearer the source—and a third wire, B, also stretched across them, but on the side close to the plate. These three wires left shadows on the plate, of which B was sharp and definite, while A and C were blurred. Two sciagraphs were taken by Mr. Robinson, one with the magnet on, and one with the magnet reversed. On subsequently superposing the two plates, with the sharp shadows of B coincident, the very slightest displacement of shadows A and C could have been observed, although those shadows were not sharp. But there was absolutely no perceptible displacement, the fit was perfect. Consequently the hypothesis of a stream of electrified particles is definitely disproved as no doubt had already been effectively done in reality by Professor Roentgen himself. But it must be noted, he stated, that the hypothesis of a simple molecular stream—not an electrified one—remains a possibility. The only question is whether such an unelectrified bombardment would be able to produce the observed effects. It must be remembered, Dr. Lodge stated, that Dr. Lenard found among his rays two classes as regards deflectibility—some much deflected, others less deflected; and it must 111be clearly understood that his deflections were observed, not in the originating discharge tube, where the fact of deflection is a commonplace, but outside, after the rays had been, as it were, “filtered” through an aluminum window. He did not, indeed, observe the deflection in air of ordinary density; it was in moderately rarefied air that he observed it, § 72a, but he showed that the variation of air density did not affect the amount, but only the clearness of the minimum magnetic deflection. The circumstance that affected the amount of the deflection was a variation in the contents of the originating or high-vacuum tube.
114. Lodge’s Experiment. Apparatus Employed. The Elect., Lon., April 10, ’96, p. 783.—With his apparatus, he was able to obtain rays sufficiently powerful to illuminate the usual fluorescent screen after passing through one’s skull. It is of interest to note about the details of the electrical apparatus (§ § 106, 109, 131, 137) used by those who experimented. The best results were obtained by a make and break of a direct primary current at a point under alcohol, the primary battery consisting of three storage cells, and the current of the primary acting on a large secondary coil. Leyden jars he considered entirely unnecessary, and he preferred direct currents to alternating currents for the primary. He did not give the exact dimensions of the primary and secondary coils, but, judging from reports of others and the author’s own experience, it is highly preferable to have what is called a very large inductorium, 15 in. spark in open air, or else the Tesla system (§ § 51, 137). There is little satisfaction in trying to perform the experiments with induction coils adapted to give only a 2 or 3 in. spark in open air.
112115. Lodge’s Experiment. X-rays Equally Strong during Fatigue of Glass by Phosphorescence. The Elect., Lon., Apr. 10, ’96.—In order to explain in what way the rays were propagated, he says that it is not as if the glass surface were a wave front from every point of which rays proceed normally, but that the glass radiates X-rays just as a red-hot surface radiates light, namely, a cone of rays starts from each point, and all the rays of each cone start in a different direction. Every point of the glass radiates the rays independently of all other points. Crookes’ Experiment (§ 58) may now be called to mind in reference to the fatiguing of the glass after phosphorescing for a while. Lodge tested the fatiguing as to the power to emit X-rays, but found that there was no such property whatever. The glass which became fatigued as to luminous phosphorescence (§ 105) was not fatigued as to the power of 113X-rays. He noticed that the phosphorescent spot became less and less bright, and yet the X-rays remained of the same power.
116. Rowland, Carmichael and Briggs’ Experiment. Area Struck by Cathode Rays only an Efficient Source When Positively Electrified. Electricity, N.Y., Apr. 22, ’96, p. 219.—Experiments carried on at the Johns Hopkins University led the above named investigators to think at first that the source of the X-rays was at the anode. Amer. Jour. Sci., March, ’96. Prof. Elihu Thomson was led to give the same opinion during his first experiments. Elect. Rev., N.Y., Mar. 25, ’96. See also § 112a. Many other experiments certify to the allegation that X-rays are certainly generated at the phosphorescent spot on the glass. § 79, 105, 107, 108, 111, 112, 113. From the experiments of Prof. Rowland, et al., the confusion is accounted for by the fact that they overlooked the electrical condition of the spot struck by the cathode rays. Prof. Rowland, et al., constructed a tube having a platinum sheet located at the focus of the concave electrode, and not connected to the anode. Although the platinum became red hot, it emitted no X-rays, but when the platinum was made the anode, there was profuse radiation of X-rays in all directions from that side of the platinum struck by the cathode rays, and no radiation from the other side. § 91. (See also Roentgen and Tesla, concerning 1/2 platinum and 1/2 aluminum and radiation therefrom.) They inferred as a final conclusion in connection with this point, “That the necessary condition for the production of X-rays is an anode bombardment by the cathode discharge.” § 113. They recognized apparently that it had been conclusively proved that X-rays radiated from the phosphorescent spot on the glass. They held that such a spot is “The induced anode formed on the glass.” § 49, at end. They did not prove this by an experiment according to the article referred to, but based it upon “The fact that the bombarding cathode rays coming in periodical electrified showers alternately raise and lower the potential of the glass, thus making it alternately an anode and a cathode. In the case of the platinum, this could not occur to the same extent.”
117. Salvioni’s Experiment. Transposition of Phosphorescent Spot. Elect. Rev., Lon., Apr. 24, ’96, p. 550; Med. Sur. Acad., of Perugia, Italy, Feb. 22, ’96. Personal interview with Prof. Salvioni in Elect. Rev., N.Y., Apr. 8, ’96, p. 181.—In order to change the location of the phosphorescent spot when desired, without a magnet, and at the same time to concentrate or intensify the source of X-rays, he placed near the same, on the outside of the tube, the hand or a metal mass connected to earth. 114The spot immediately jumped to the other side of the tube, § 49, near centre, and to all appearances was smaller and brighter. Elster and Geitel had performed similar experiments at an earlier date. (See Wied. Ann., LVI., 12, p. 733, also Elect. Eng., about April, ’96.) They carried on the most minute investigations as to the deflection of the cathode rays by an outside conductor. Tesla had also noticed a similar deviation. See Martin’s Tesla’s Researches. He used alternating currents as described in his system in § 51. Elster and Geitel used the Tuma Alternating system. (See Wied. Ann., Ber. 102, part 2A, p. 1352, ’94.) The source from which Salvioni’s description was taken had no sketch, therefore the diagram made by Elster and Geitel is reproduced. See Fig. 1. The cathode was aluminum and was connected to one terminal of the transformer. The anode was connected to earth, and also was the other terminal. Upon bringing the hand or other conductor connected to earth to the phosphorescent spot, the cathode rays deviated and the spot jumped over to the other side. § 50. The anode was a ring surrounding the leading-in wires of the cathode, and the two leading-in wires were surrounded by glass. It may be asked why the cathode rays bent downward in the first place? Elster and Geitel found that they were thrown thus in view of the nearness of some neighboring object connected to earth. To overcome the action of surrounding objects, the tube was surrounded by a ring as shown in Fig. 2. However, the rays were still sensitive to objects well connected to earth, and when brought quite close to the tube.

Figs. 1 and 2.
117a. Hammer and Fleming’s Molecular Sciagraph, within a Vacuum Tube. (Citations below.)—In view of the overwhelming evidence concerning the generation of X-rays by the impact of cathode rays, within a high vacuum upon the glass or material which preferably forms the anode, it becomes appropriate, it is thought, to review the state of this department of science, in order to arrive a little more closely at the relations which exist between phenomena of low and high vacua. With the former, in that condition in which striae are formed, permanent 115black bands or deposits are produced upon the surface of the glass; the motion of the particles, therefore, appearing to be in planes at right angles to the line joining the anode and cathode. § 40. That the striae should touch the walls of the tube seems to be necessary for the production of the deposit. § 44. With a high vacuum, the direction of the cathode rays may be any that one desires, it being only necessary to shape the cathode properly, on the principle that the rays radiate normally from the surface. It is known that the radiation is normal as much from the position of the deposit as from that of the phosphorescent spot. It is certain that they are rectilinear. § § 57 and 58. The phosphorescent spot becomes always, sooner or later, when occurring upon the same part of the glass, the location of a deposit from the cathode (§ 123), even when the cathode is aluminum. § 123. The deposit is not the cause of the fatigue of the glass. § 58. Puluj verified this. A wheel was made to rotate by the radiations from the cathode, and therefore it is highly probable that the motion of the molecules, which caused the deposit, is the force that made the wheel rotate. § 58a. Why does it not follow that with increase of E. M. F. the particles are thrown with such rapidity that upon striking the proper surface (§ 80), X-rays are generated, but that they are not generated when the velocity of the molecules is insufficient. § 61b, p. 46. Attention is now invited to a phenomenon which illustrates that a permanent sciagraph of objects may be impressed upon the inner surface of a vacuum tube, by the deposit of molecules of one of the electrodes. Refer, therefore, to the figure on page 30, “Hammer and Fleming’s Molecular Sciagraph.” As will be seen from further explanation and from the picture itself, the sciagraph a b is made because of the projection, in rectilinear lines, of molecules of carbon or metal, from one of the electrodes, or at least from one more than the other. One leg of the carbon, being in the way of the other, causes a less deposit to be produced upon the glass at the intersection of the plane of the horse-shoe filament and the wall of the vacuum tube. Electrodes exist because the filament is of such a high resistance as to produce a difference of potential between the two straight lower portions of the filament. Mr. William J. Hammer possesses a remarkable faculty for observing phenomena often overlooked by others. He first observed a molecular shadow in 1880 and made records of his observations in the Edison Laboratory note book. Since that time he has examined over 600 lamps, which were made at various periods during thirteen 116or fourteen years, by twelve different manufacturers. (Trans. Amer. Inst. Electrical Eng., Mar. 21, p. 161.) Every one, more or less, exhibited the molecular shadow. It is a principle, therefore, that if the carbon filament has both legs in the same plane, a sciagraph of one of them will be produced. As the shadow is on one side of the bulb only, the molecules fly off from only one electrode, viz., the cathode. By means of photography, the effect is increased because of certain well-known principles. The figure heretofore referred to is taken from a photograph, but, of course, does not represent the sciagraph as well as the original photograph, in view of the loss of effect by re-production by the half-tone process. For further theoretical considerations, see the Institute paper referred to, where the matter was discussed by Profs. Elihu Thomson, Anthony and others. Independently of Mr. Hammer’s discovery, Prof. J. A. Fleming, professor of electrical engineering in the University College, London, England, discovered and studied the matter, and presented it before the Phys. Soc. of London, appearing about 1885 (from memory). The name “molecular sciagraph” is given by the author because it is an accepted explanation that the deposit is due to either molecules or atoms of the electrode, given off by evaporation (page 46, lines 5 to 10), or electrical repulsion (§ 61a, lines 22 to 25), or, as some hold, by mere volatilization by the intense heat of incandescence, or one or more combined; but electrical repulsion certainly has something to do with the rectilinear propagation, for the molecules are charged according to § 4.

118. Edison’s Experiments. Characteristics of Discharge Tube, Photographic Plates, Electrical Apparatus, Fluorescence, Etc. Elec. Eng., N.Y., Feb. 19, ’96; Mar. 18 and 25; Apr. 1, 8, 15 and 29, ’96. X-Rays Begin Before Striae End.—The reader may remember a former section, § 10, pointing out that striae were usually obtainable without very high vacua, and that phosphorescence of the glass occurs only with high vacua. § 54. In carrying the vacuum up higher and higher, Edison observed that feeble Roentgen rays were detected before the striae ceased. Prof. Elihu Thomson independently performed a like experiment and found that the Roentgen rays could be obtained even when the vacuum was so low as to produce striae. (Elec. Eng., N.Y., Apr. 15, ’96.) Victor Chabaud and D. Hurmuzescu also obtained X-rays from a vacuum .025 mm., being lower than Crookes employed, which was at a maximum .001 mm. (L’Industrie Elect., Paris, May 25, ’96. From trans. by Louis M. Pignolet.)
119. Reason Why Thin Walls are Better Than Thick. X-Rays and Post-Phosphorescence.—This may be understood by explanation of the discharge tube in Fig. 1. In one experiment, the portion struck by the cathode rays, namely B, was made 1/8 inch thick. It became soon hot and very luminous and melted, § 61, but the X-rays were weak. When blown thin, (§ 83) however, the glass remained cool and the X-rays were much stronger. What is known on the market as German glass (phosphoresces green, § 55, at centre) was found more permeable than lead glass, the thickness of the walls being the same in both cases. There were no lingering X-rays from after-phosphorescence, (§ 54, at end) or, if any, could not be detected by the sciascope. The photographic test would be objectionable because of the brief duration. Prof. Battelli and Dr. Garbasso, of Pisa, made a very sensitive test in this connection, proving by the discharge of an electrified body (§ § 90 and 90a) that feeble X-rays were emitted after the current was cut off from the discharge tube. (From trans. by Mr. Pignolet.)
120. To Prevent Puncture of the Discharge Tube by Sparks.—In the illustration, Discharge Tube Fig. 2. shows a suitable type. It is drawn to scale, showing the correct proportion of the length to the diameter. The shaded ends represent tinfoil on the outside and connecting with the leading-in wires, the same preventing puncture of the glass by the spark. They may be caused to adhere by shellac or similar glue. In place of the metallic coating detached supplementary electrodes may be employed, as seen in the illustration marked “Discharge Tube Fig. 3.” The power of the X-rays was increased, being due, it 119was thought, to the fact that the construction embodied the combination of internal and external electrodes. § 121.
121. Variation of Vacuum by Discharge and by Rest.—Prof. Pupin was among the first to test the efficiency of external electrodes for generating X-rays. Independently of the quality of the glass and of the kind of pump and of the presence or absence of phosphoric anhydride, the following peculiarities were noticed, which Edison attributed to a kind of atomic electrolysis. § 47. 80 per cent. of the lamps exhibited the phenomena as follows: First, such a high vacuum was obtained by the pump that the line spectrum disappeared and pure fluorescence and generation of X-rays at a maximum occurred. The lamp was then sealed off. After three or four hours of rest, the vacuum deteriorated, so that striae and other characteristics of low vacuum were obtained when connected up in circuit, but upon continuing the current, the high vacuum gradually came back, the line spectrum vanished, and suddenly X-rays were generated. Again the bulb was left at rest for 24 hours, after which X-rays could not be generated until the discharge had been continued for 4-1/2 hours.
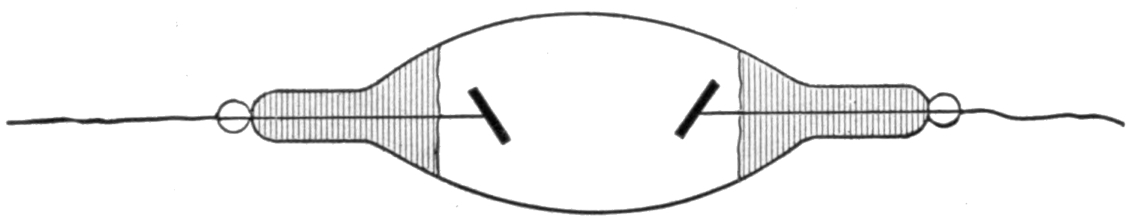
Discharge Tube, Fig. 2. § 120.
122. External Electrodes Discharge through Higher Vacuum than Internal.—A vacuum that was so high that no discharge took place with internal electrodes was made luminous by the use of electrodes on the outside of the glass bulb. Then he made the vacuum so high that even with a 12-inch spark from Leyden jars, no discharge took place with external electrodes, and the tube was dark, this part of the experiment indicating another limit at which an extremely high vacuum is not a conductor and appearing to overthrow, as Edison intimated, Edlund’s theory that a vacuum is a perfect conductor. § 25.
123. Deposit on Glass from Aluminum Electrode.—It has always been common to employ aluminum for electrodes in vacuum tubes, on the ground that no deposit took place, and therefore no blackening, nor whitening of the glass wall. § 40. Edison observed also that no blackening was visible, but stated that his glass blower, Mr. Dally, upon breaking the bulb and submitting the interior surface of the glass to an oxydizing 120process, the oxide of aluminum was so thick as to be opaque to light. With magnesium, also, a mirror was produced, of a lavender color, by transmitted light. In the case of aluminum, he was able to obtain a visible spot at the phosphorescent portion, but only after a great many hours of use. See cut from a photograph of a discharge tube used for several months by Prof. Dayton C. Miller, and having a heavy aluminum deposit opposite the aluminum cathode. With the increase of the deposit, the power of the X-rays diminished, but, he thought, not on account of the absorption, but because, “through lack of elasticity at the surface.”
Discharge Tube, § 123.
124. Fluorescent Lamp. In an English patent of ’82, granted to Rankin Kennedy, there is described a vacuum bulb in which the electrodes are covered with fluorescent or phosphorescent substances, intended for the purpose of obtaining greater candle power by impact of cathode rays upon anode of platinum, covered with alumina or magnesia. Edison coated the inner wall of the discharge tube, for generating X-rays, with calcic tungstate in the crystalline form. The luminosity, when measured, amounted to about 2-1/2 C. P. As to the efficiency, he stated that this was accomplished “with an extremely small amount of energy.” Such a coating was found to weaken the X-rays radiated therefrom, which, of course, was natural, because they had been converted into phosphorescent light. The spectrum showed strongly at the red line, thereby suggesting the reason why the light was of a pleasant character.
121124a. Piltchikoff’s Experiment. Greater emission of X-rays by a tube containing an easily fluorescent substance. Comptes Rendus, Feb., 24, ’96. From trans. by Mr. Louis M. Pignolet. As the X-rays emanate from the fluorescent spots on the glass of the discharge tube, he reasoned that more powerful effects would be obtained by replacing the glass by a more fluorescent material. He therefore tried a Puluj tube and found that it shortened the time necessary for taking a photograph in a “singular” degree. Experiments of others have certainly shown that as phosphorescence decreases with increase of vacuum, the X-rays increase to a certain maximum, § 105. Let it be noticed however, that this does not prove that with the same vacuum, an increase of phosphorescence by a superior phosphorescent material of equal thickness would not increase the power of the X-rays. The best way to determine such points, is to go to extremes. Edison applied so much easily phosphorescent material (calcic tungstate) to the inside of the discharge tube, that much light was radiated, but only feeble X-rays. On the other hand, without any of the tungstate, the rays were strong, § 124. Experiments generally tend to prove that it depends upon the chemical nature of the material rather than its phosphorescing power, in other words upon the permeability. § 119, near end.
125. Electrodes of Silicon Carbide. (Carborundum.) Edison called attention to Tesla’s discovery that this substance is a good conductor for high tension currents. Its advantages for electrodes in the discharge tube are its high conductivity, no absorbed nor released gas bubbles, and its infusibility and non-blackening power of glass even when the voltage was increased to a point where the glass melted.

126. Chemical Decomposition of the Glass Bulb. During the generation of the X-rays the sodium line of the spectrum appeared in the spectroscope, thereby indicating decomposition of the glass. With combustion tubes the glass gave the weakest soda line, while lime soda glass gave the strongest, and was most permeable to the X-rays. “The continuous decomposition of the glass makes it almost impossible to maintain a vacuum except when connected to the pump and even then the effect of the current is greater in producing gas than the capacity of the pump to exhaust, but the ray is very powerful.” It is supposed that for this reason, as well as for others easily apparent that Edison as well as other experimenters have always carried on their investigations with the discharge tube permanently connected to the pump. The next best thing is to let the tube contain a stick of caustic potash for maintaining an exceedingly 123high vacuum. By gradually heating this, the desired degree of vacuum can be obtained. § 54.
127. Sciagraphs. Duration of Exposure Dependent Upon Distances. With the given discharge tube, he obtained sciagraphs at a distance of 3/8 inch from the phosphorescent spot in one second, a vulcanized cover being between; at two ft. distant the time was 150 sec.; at three ft., 450 sec.; the opaque plate being interposed each time. Consequently “Roughly, the duration of exposure may be reckoned as proportional to the square of the distance.”
128. Difference Between X-rays and Light Illustrated by Different Photographic Plates. Time of Exposure. The rapid plate for light gave not the deepest images by X-rays. Several different kinds of small sensitive plates were laid side by side. A sciagraph of a metal bar was taken upon them all simultaneously. In this way, he obtained the result, whereby it would appear preferable to employ the mean rapid plate for the purpose of obtaining sciagraphs. On account of the opacity of platinum, it occured to E. B. Frost, (Sci., N.Y., Mar. 27, ’96,) to try platinum photographic paper of the kind used for portraits, but such paper (intended for long exposures in printing in sunlight) was far too lacking in sensitiveness to produce any effect.
128a. Georges Meslins insured a reduction of time for taking sciagraphs by the deflection of the cathode rays by means of a magnetic field. Comptes Rendus, March 23 and 30, 1896. From trans. by Louis M. Pignolet. The method consists in using a permanent or electro-magnet to create a magnetic field perpendicular to the cathode rays in the tube. By this means, the active fluorescent spot on the tube is condensed, and the intensity of the X-rays generated there is increased. Another advantage is that, when the active part of the tube becomes inactive owing to the formation of a light brown deposit upon it, another part can be used by very slightly altering the position of the magnets. Thus, each time a new part of the tube can be used. The magnetic field must not be uniform but must have a suitable variation to produce the desired concentration of the cathode rays.
A. Imbert and H. Bertin-Sans’ Experiment. (Comptes Rendus, March 23, ’96. (From trans. by L. M. P.) They shortened the time by use of a magnet.
James Chappin’s Experiment. (Comptes Rendus, Mar. 30,’96. (From trans. by L. M. P.)—Claimed priority in having shown publicly, on Feb. 19, a sciagraph of a hand, marked “Photograph obtained by concentration of the cathode rays, by means of a 124magnetic field.” The increase of the intensity of the X-rays obtained by this means was in the proportion of 8 to 5, as measured by the time of fall of the leaves of a Hurmuzescu electroscope.
Prof. Trowbridge, of Harvard University, in a lecture, gave an interesting review (Western Elect., Feb. 29, ’96) of the length of time required in the early days of photography. Improvements are being made whereby the duration required in sciagraphy becomes less and less. In 1827, by heliography, 6 hours’ exposure was necessary; in 1839, by daguerreotype, 30 minutes; in 1841, by calotype, 3 minutes; in 1851, by collodion, 10 seconds; in 1864, by collodion, 5 seconds; in 1878, by gelatine, 1 second. The author remembers the photographs for use in the Edison kinetoscope were taken at the rate of 20 per second. The focus tube brings the time of exposure in behalf of X-rays down to a matter of seconds instead of minutes. For an admirable review of authorities, facts and theories relating to the causes of the darkening of photographic plates by light, see Cottier, in Elect. World, N.Y., May 23, ’96.
129. Size of Discharge Tube to Employ for Given Apparatus.—A small tube required but a small E. M. F., and therefore should be employed with a small induction coil. The greater the distance of the sensitive plate and the object, considered together, from the discharge tube, the sharper the shadow. In short exposures, the tube should be small and at a short distance.
130. Preventing Puncture at the Phosphorescent Spot.—In experiments where he employed a flat cathode, a very thin pencil of rays of increased power came from the exact centre, and in two or three seconds made the glass red hot at the centre of the phosphorescent spot. Immediately, the atmospheric pressure perforated the bulb. This occurred several times. He stated that “the best remedy is to permit the central ray to strike the glass at a low angle; this greatly increases the area and prevents the trouble.” Edison.
Mr. Ludwig Gutmann furnished a translation of a note by Prof. Walter König, found in Eleck. Zeit. of May 14, ’96, relating to this same subject matter. Recognizing that the sharpness of the outlines is the most important requirement in connection with sciagraphy, and that if the rays start from a large surface the impressed shadows will be uncertain in configuration, and noticing, as Edison and Tesla did, § 130, the frequent destruction of the tube at the place where the rays were concentrated to a focus, he placed over the inner surface of the glass, 125aluminum foil for distributing the heat over a larger area, at the same time causing radiation of X-rays from a single point. The focus tube outweighs this in importance. § 91.
131. Electrical Dimensions of Apparatus. The best kind of instruction for the student in reference to equipping a plant is to follow the construction employed by those who have been successful. § § 106, 109, 114, 137. Edison used the usual incandescent-lamp current, voltage at 110 to 120 volts, current being continuous, but not connected directly to the induction coil, there being a bank of eight to twenty 16 candle power incandescent lamps arranged in parallel. The interrupter for the primary consisted of a rotating wheel in appearance like a commutator of a dynamo, and was rotated rapidly by a small electric motor, making about 400 interruptions per second, and so constructed that the circuit was closed twice as long as it was open. A sudden interruption was caused by an air blast playing at the point of make and break, the use of which made that of a condenser needless. § 3. The discharge tube terminals were connected respectively and directly to those of the secondary. Prof. Pupin, Columbia Univ. N.Y. (Lect. N.Y., Acad. Sci., April 6, ’96, and Science, N.Y., April 10, ’96) gave valuable and practical instruction concerning the apparatus, which the author witnessed. “A powerful coil was found indispensable for strong effects and satisfactory work. The vibrating interrupter is too slow and otherwise unsatisfactory, and it was replaced by a rotary interrupter, consisting of a brass pulley, 6 inches in diameter and 1-1/4 inches in thickness. A slab of slate 3/4 inch thick was inserted and the circumference was kept carefully polished. This pulley was mounted on the shaft of a Crocker-Wheeler 1/8 H. P. motor giving 30 revolutions, and, therefore, 60 breaks per second. Two adjustable Marshall condensers of three microfarads each were connected in shunt with the break, and the capacity adjusted carefully until the break-spark was a minimum and gave a sharp cracking sound. Too much capacity will not necessarily increase the sparking, but it will diminish the inductive effect which is noticed immediately in the diminished intensity of the discharge. A powerful coil with a smoothly working rotary interrupter will be found a most satisfactory apparatus in experiments with Röntgen radiance.” § 106, 109, 114, 131, 137.
132. Salts Fluorescence by X-rays. See also, Elect. Rev., N.Y., April 19, ’96, p. 165. Edison examined over 1800 chemicals to detect and compare their fluorescent powers if any, under the action of X-rays first transmitted through some opaque 126material such as thick cardboard. Of all these, calcic tungstate by measurement, fluoresced with six times the luminosity of barium platino cyanide, which was referred to in connection with Roentgen’s experiment. Other authorities agree as to its great sensitiveness. In making this comparison, it was assumed that the power of the X-rays varied inversely as the square of the distance from the discharge tube. Between the two above chemicals came strontic tungstate. Baric and plumbic tungstate scarcely fluoresced. Salicylate of ammonium crystals equalled the double cyanide of platinum and barium, and differed therefrom in that the fluorescence increased with the thickness of the layer of crystals up to 1/4 of an inch, showing great fluorescing power and low absorptivity. This experiment showed that the best fluorescent materials were not necessarily the salts of the heaviest metals, like platinum. It is assumed that the reader knows the difference between phosphorescence and fluorescence, but the dividing line is so difficult in some cases and the one not being distinguished from the other by experimenters, that the author has used the same words as the experimenters, although he admits that fluorescence is often meant where phosphorescence is stated, and vice versa. An anomaly presented itself as to rock salt, which although transparent to light yet powerfully absorbed X-rays and was strongly fluoresced thereby. Again, fluorite which is transparent to light, fluoresced strongly with the X-rays, and under their action became brighter and brighter and continued after cutting off the X-rays, the material therefore, being highly phosphorescent, the light enduring for several minutes. Upon watching the phosphorescence of fluorite, the same penetrated the plate very slowly to the depth of one-sixteenth of an inch, but beyond that depth there was complete darkness. The only other truly phosphorescent substance noticed was calcic tungstate, especially in thick layers, so that the shadow of the bones of the hand remained thereon for a minute or two upon cutting out the discharge tube from the circuit. Some chemicals, within a dark box and very close to the discharge tube, phosphoresced by giving spots here and there, but they did not phosphoresce at a greater distance, and the light was probably not due to the X-rays. Edison attributed the result directly to the “electrical discharge.” The list is as follows: ammonium sulphur cyanide, calcic formate, and nitrate, ferric citrate, argentic nitrate, calcic and iron citrate, soda, lime, “zinc, cyanide” (perhaps this means cyanide of zinc), zinc hypermanganate, and zinc valeriate. The salts of the following metals did not fluoresce under the influence of the 127X-rays. Aluminum, antimony, arsenic, boron, beryllium, bismuth, barium, chromium, cobalt, copper, gold, iridium, magnesium, manganese, nickel, tin, and titanium.
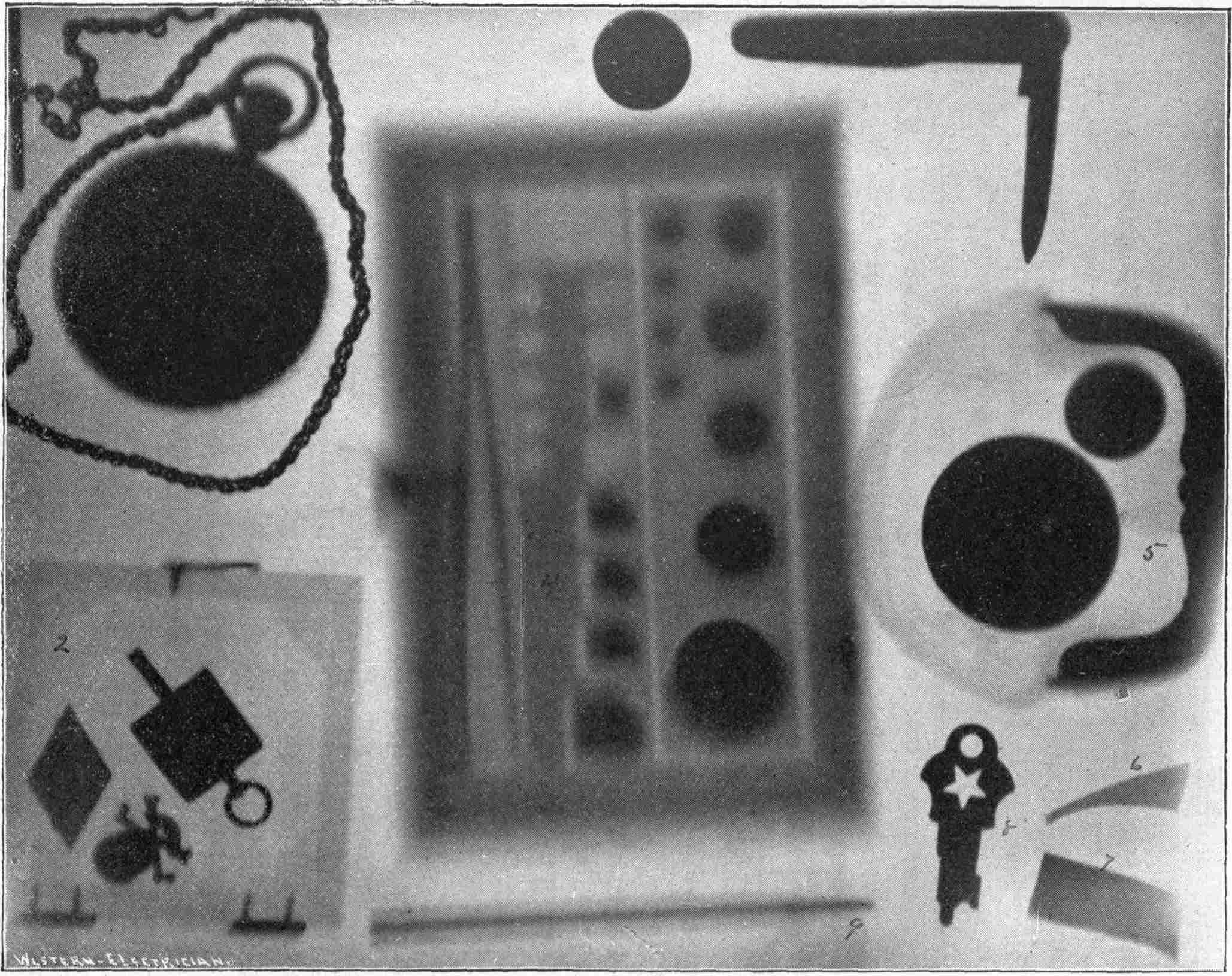
Roentgen Rays at the University of Minnesota.
1. Watch and chain.
2. College badges in mahogany box.
3. Copper coin.
4. Weights in heavy velvet-lined mahogany box; blank space contains aluminum.
5. Coins in inner pocket of heavy seal purse.
6 and 7. Colored glass.
8. Key
9. Lead-pencil.
West. Elect., Mar. ’96.
Edison stated that the following substances were among those which fluoresced more or less under the action of the X-rays. Mercurous chloride, mercury diphenyl, cadmic iodide, calcic sulphide, potassic bromide, plumbic tetrametaphosphate, potassic iodide, plumbic bromide, plumbic sulphate, fluorite, powdered lead glass, pectolite, sodic cressotinate, ammonic salicylate, and salicylic acid. Compared with the above, the following fluoresced less. Powdered German glass, baric, calcic and sodic fluorides, sodic, mercuric, cadmic, argentic and plumbic chlorides, plumbic iodide, sodic bromide, cadmic and “cadmium, lithia bromide, mercury, cadmium sulphate,” uranic sulphate, phosphate, nitrate, and acetate, molybdic acid, dry potassic silicate, sodic bromide, wulfenite, orthoclase, andalucite, herdinite, pyromorphite, apatite, calcite, danburnite, calcic carbonate, strontic acetate, sodic tartrate, baric sulphobenzoic, calcic iodide, and natural and artificial ammonium benzoic. Not one of all the 1800 crystals and precipitates fluoresced through a thick cardboard under the influence of the arc light, 16 inch spark in air, a vacuum tube so highly exhausted that a 10 inch spark left it dark, nor the direct rays of the sun at noon time. As calcic tungstate was phosphorescent by friction, he theorized that the X-ray is a wave due to concussion.
Flame sensitive to X-rays. Edison stated that his assistants submitted the sensitive flame and the phonographic listening tube to the action of the X-rays, and found that they were responsive thereto.
133. X-rays Apparently Passed Around a Corner. Referring to the figure “X-ray Diffusion Fig. 1”, p. 129, it will be noticed that there were three principal elements. First a discharge tube, then a thick steel plate and then a sciascope, all arranged in the proportion indicated in the figure, where the sciascope was within six inches of the edge of the plate, “well within the shadow” thereof. § 69. Fluorescence was seen under these conditions. When the sciascope was directly behind the middle of the plate and opposite the discharge tube, there was no fluorescence, showing that the plate was thick enough to cut off all the rays and therefore the energy must have traveled in two directions for some reason or other.
Prof. Elihu Thomson remarked concerning this experiment that he considered, in view of some experiments of his own, on diffusion and opalescence (§ 103), that the sciascope was luminous 129in view of reflection (§ 146) of the X-rays from various objects in the room, as from the walls and floor of the room, tables, metal objects, electrical apparatus and so on. Theory admits the property of diffraction, which would cause the rays to turn around the edge of the plate, according to the principles of diffraction of light, provided the X-rays were due to transverse or longitudinal or any vibrations. See Elect. Eng., N.Y., April 15, p. 378.
While Edison generally devotes his energy to actual experiments and dealings with facts and principles, rather than with theories, yet, in this instance, he merely suggested that the fluorescence under the conditions named might indicate that the propagation of X-rays was similar to that of sound in air, the wave being of exceedingly short length. He referred to Le Conte’s experiment of ’82 (see Phil. Mag., Feb. ’82), where an experiment of a somewhat similar nature was performed in connection with the propagation of sound.

X-Ray Diffusion, Fig. 1, § 133.
Prof. William A. Anthony (see Elect. Eng., Apr. 3, ’96, p. 378) held that the Le Conte experiment did not warrant Edison’s conclusion, for the experiment of Le Conte showed comparatively sharp sound shadows; for even at a distance of twelve feet there was no apparent penetration within the geometrical boundary. He referred to Stine’s, § 110. Scribner and M’Berty’s, § 111, as upholding rectilinear propagation. While he did not explain what the Edison result was due to, yet he argued that the cause was other than that ascribed by Edison. In this connection, the author performed an experiment (Elect. Eng., Apr. 22, ’96, p. 409) to substantiate that X-rays were propagated through such a high vacuum that it was necessary 130to have electrodes within 1/8 of an inch of each other, in order to obtain a discharge with a coil that gave 15 in. spark in open air. The experiment consisted in casting the shadow of an uncharged tube upon the screen of a sciascope. The shadows of the wire forming the electrodes within the vacuum were produced very sharply, while the glass tube was faintly outlined. Inasmuch as the shadows of objects within the vacuum tube were obtained, therefore the X-rays must have passed through the evacuated space. Sound and X-rays are therefore dissimilar. The shadows were as sharp and as dark as those made by similar wires in open air. In this connection, see also Lenard’s experiment, § 72, showing that external cathode rays were also transmitted by a vacuum in a “dead” tube. Roentgen’s experiment showed that X-rays from a mass located entirely within the vacuum in the discharge tube radiated X-rays into the outside atmosphere. § 91. This experiment would hardly prove, however, that X-rays, after having been liberated in open air, would pass through a second vacuum space, because there may have been some X-rays, generated at the surface of the glass in Roentgen’s experiment, struck by those rays which radiated from the mass at the centre of the vacuum space. Did not Lenard and Roentgen experiment with the same radiant energy? The author answers, yes. § 77.
134. Permeability of Different Substances. Lenard § 68. determined the permeability of several substances to cathode rays. Roentgen also the same in regard to X-rays. § 82 and 83. Others have made comparisons. From the sciagraph made by Edison, the following classification is made, each sheet of material being about 1/32 inch thick. The most opaque were coin silver, antimony, lead, platinum, bismuth, copper, brass, and iron, which were about the same as one another. Slate, ivory, glacial phosphoric acid shellacked, and gutta percha, were about the same as one another and less than the above. Aluminum, tin, celluloid, hard rubber, soft rubber, vulcanized fibre, paper, shellac, gelatine, phonographic cylinder composition, asphalt, stearic acid, rosin, and albumen, were about the same as one another and less than the above group, as to permeability.
The accompanying picture, p. 6, marked Terry’s Sciagraph, Fig. 1, is a sciagraph of pieces of different materials named as in the following list, taken by Prof. N. M. Terry of the U.S.N.A., see also p. 127. “1, rock salt, 0.6 inch thick; 2, cork, 0.4 inch thick; 3, quartz, 0.45 inch thick, cut parallel to optic axis; 4, verre trempe, 0.4 inch thick; 5, glass, 0.7 inch thick; 6, chalk; 7, Iceland spar; 8, mica, very thin; 9, quartz, over a square piece of 131glass; 10, aluminum foil, [a] four thicknesses, [b] two thicknesses, [c] one thickness; 11, platinum foil; 12, tourmaline; 13, aragonite; 14, paraffine, 0.4 inch thick. 15, tin foil, [a] one thickness, [b] two thicknesses, [c] three thicknesses; 16, rubber insulated wire; 17, electric light carbon; 18, glass, 0.32 inch thick; 19, alum., 1.4 inch thick; 20, tourmaline; 21, gas coal; 22, bee’s wax; 23, pocket-book, 10 thicknesses of leather; 24 coin in pocket-book; 25, key in pocket-book; 26, machine oil in ebonite cup; 27, ebonite, 0.25 inch thick; other samples have given very faint shadows like wood and leather; this was polished; 28, wood, 0.2 inch thick; 29, steel key.” Elect. Eng., N.Y.
134a. Hodges’ Experiment. Illustration of Penetrating Power of Light. Elec. Eng., N.Y., March 4, ’96. Attention has been invited in the scientific press to the penetrating power of heat rays and of light rays of low refrangibility. In conjunction with this, let it be remembered that the photographic plate has the property of being impressed practically, only by rays having a higher refrangibility than red. It would be natural, therefore, to conclude that if the spectrum could be turned around, the photographic impression might be produced through opaque bodies. This perhaps, was the kind of reasoning which prompted Mr. N. D. C. Hodges, formerly editor of Science, to perform an experiment, the gist of which consisted in attesting the permeability of rays of light which had been passed through fuchsine. Christiansen, Soret and Kundt performed experiments with an alcoholic solution of this material and found that the order of the colors in the spectrum was somewhat reversed, namely, violet was the least refracted, then red, and then yellow, which was the most refracted. Mr. Hodges used a pocket kodak, carrying a strip for twelve exposures. This camera was placed in a closely fitting pasteboard box. Thus protected, some portions of the film were exposed to sunlight, so far as it could penetrate the end of the pasteboard box, while other exposures were made with a prism, on the end of the box, containing an alcoholic solution of fuchsine. The portions of film exposed to the anomalous rays produced by the fuchsine solution were fogged, while the control experiments with ordinary light showed none. The anomalous rays must have penetrated the pasteboard, and probably the wood and leather of which the camera was made.
135. Penetrating Power of X-rays Increased by Reduction of Temperature. § 23 and 72b at end. Among the hundreds of ideas that occured to Edison in connection with Roentgen ray tests was that concerning what might happen by cooling the 132discharge tube to a very low temperature. As before, he maintained the tube in connection with the air pump so as to be able to vary the vacuum. The reduction of temperature was obtained by means of ice water. Of course the bulb could not be placed in the water itself on account of trouble which would occur from electrolysis, therefore, the discharge tube was immersed in a vessel of oil, § 13, which in turn was surrounded by a freezing mixture. The vessel was a stout battery jar 14 inches high, eight inches in diameter with glass walls 5/10 of an inch thick. The oil employed was paraffine. The refrigerating jar was 12 inches high and 12 inches in diameter and the glass wall thereof, 3/8 inch thick. He tested the difference in the power of the rays by first noticing the thickness of steel that was not penetrated by the rays generated from the tube while in air. Crucible steel 1/16 of an inch thick did not transmit rays sufficiently to illuminate the sciascope, and yet with the use of oil and reduction of temperature, and after the rays had passed through two thicknesses of glass as well as through the oil and ice water, the sciascope was made luminous by rays after passing through a plate of steel of double the thickness, i.e. 1/8 in. thick. See in this connection, Tesla’s experiment, § 135, where powerful rays were obtained by immersing the discharge tube in oil. Accounts of these two experiments were published simultaneously. Tesla attributed the idea of this use of oil to Prof. Trowbridge of Harvard University, who showed that a discharge tube immersed in oil is adapted to the generation of X-rays of increased penetrating power. See cut at p. 135.
Non-Reflection of X-rays. (Elect. Eng., Feb. 19, ’96, p. 190. Apparently extracted from the daily press.)—That the X-rays were only slightly reflected (Roentgen, § 81., and even when very powerful (Tesla, § 146., was determined in a severe manner by Edison. The first experiment consisted in employing a funnel 8 inches long and 3/4 inch at the smaller end. The discharge tube was in the larger end, and the photographic plate across the smaller end. After experiment and development, the plate showed overlapping circular images, which would indicate reflection from the inner surface of the funnel. This may have been due to a jarring vibration of the funnel. Therefore, he carried the experiment further by using a funnel 9 feet long. The plate did not indicate any signs of reflection, as it merely became generally fogged. The material of the tube is not named, but if of brass or other impermeable metal, it is thought that his experiment would have shown a result agreeing with that of others herein. Again, the reporter may have 134been in error. Also, the rays may have been very weak, as the experiment was performed when Edison first started to investigate the subject.
136. X-rays Not Yet Obtainable from other Sources than Discharge Tubes.—Edison exposed covered plates to the direct sun-light at noon for three or four hours; no photographic impression; also to electric sparks in open air, of twelve or more inches in length; no clouding even of the photographic plate.
Profs. Rowland et. al., of the Johns Hopkins University, in a contribution to Electricity, Apr. 22, ’96, p. 219, confirmed this point by stating: “As to other sources of Roentgen rays, we have tried a torrent of electric sparks in air from a large battery, and have obtained none. Of course, coins laid on or near the plate, under these circumstances, produce impressions, but these are, of course, induction phenomena.” (See Sandford and McKay’s Fig. p. 20). “As to sun-light, Tyndall, Abney, Graham, Bell and others have shown that some of the rays penetrate vulcanite and other opaque objects.” Poincaré, at an early date, advanced the hypothesis that X-rays are due to phosphorescence, whether produced by electrical or other means. Elect. World, Digest., Mar. 28, ’96, p. 343, where it is also stated that Chas. Henry thought a certain experiment of his own was in favor of the hypothesis. The experiment was performed with a phosphorescent material which had been exposed to the light and then brought into darkness. Niewengloswski inferred, from an experiment, that phosphorescent bodies increase the penetrating power of sun-light. Tesla admitted the possibility of the radiation of X-rays from the sun. In an article describing important experiments in the Elect. Rev., N.Y., Apr. 22, ’96, p. 207, he stated: “I infer, therefore, that the sun-light and other sources of radiant energy must, in a less degree, emit radiations or streamers of matter similar to those thrown off by an electrode in a highly exhausted enclosure. This seems to be at this moment still a matter of controversy.” Roentgen, in his first announcement, showed that the phosphorescent spot was the source of the X-rays. § § 79 and 80. All the different opinions and theories, therefore, indicated that phosphorescence by sun-light might possibly emit X-rays. Probably few had sufficient belief in the matter, one way or the other, to try the experiment in an extreme manner. The author was curious to prove the question, but he only obtained negative results. It cannot be conceived how the matter could have been more severely tested, for he concentrated the light of the sun nearly to a focus by a large lens, namely 5 in. in diameter, together with 135a reflecting funnel. The maximum phosphorescence was therefore obtained by placing suitable chemicals at the opening in the funnel. The sciascope showed absolutely no X-rays present. Photographic plates were not in the least acted upon, even after hours of exposure, the same having opaque covers of aluminum. See Elect. Eng., N.Y., Apr. 8, ’96, p. 356. If X-rays are emitted from the sun, they are all absorbed by the atmosphere of the earth, or are overcome by some other force.
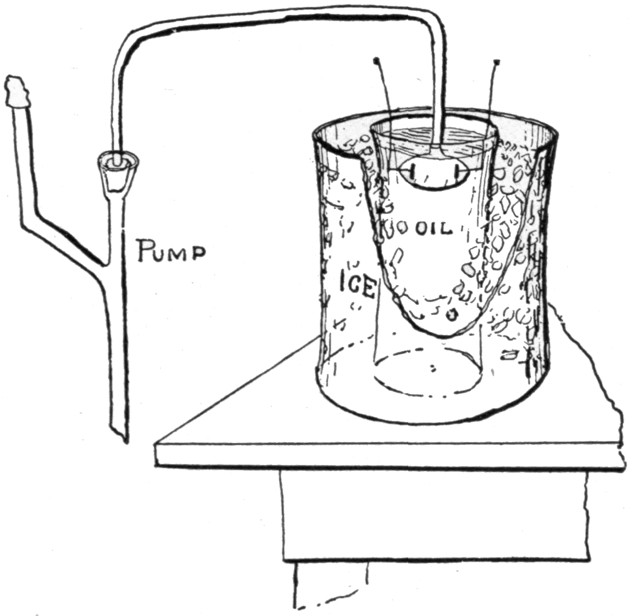
Cooling Discharge Tube. Edison. § 135.
137. Tesla’s Experiments. Elec. Rev., N.Y., March 11, ’96, page 131, March 18, page 147, April 1, page 171, and April 8, page 183. Kind of Electrical Apparatus for Operating Discharge Tubes for Powerful X-rays. § 106, 109, 114, 131. The experiments performed by Nikola Tesla were particularly noteworthy for the magnitude and intensity of the rays generated by his apparatus, under his skilful manipulation of the adjustments and circuits particularly as to resonance. The remarkable results that he obtained are not surprising when we learn that he employed his well-known system for producing exceedingly enormous potential and unusually high frequency. § 51. The primary electrical generator as he indicated and as apparent from his system referred to in the above section, could be either a direct or alternating current generator, or other form. If the first is employed, of course an interrupter is necessary in order that there may be a current induced in the secondary.
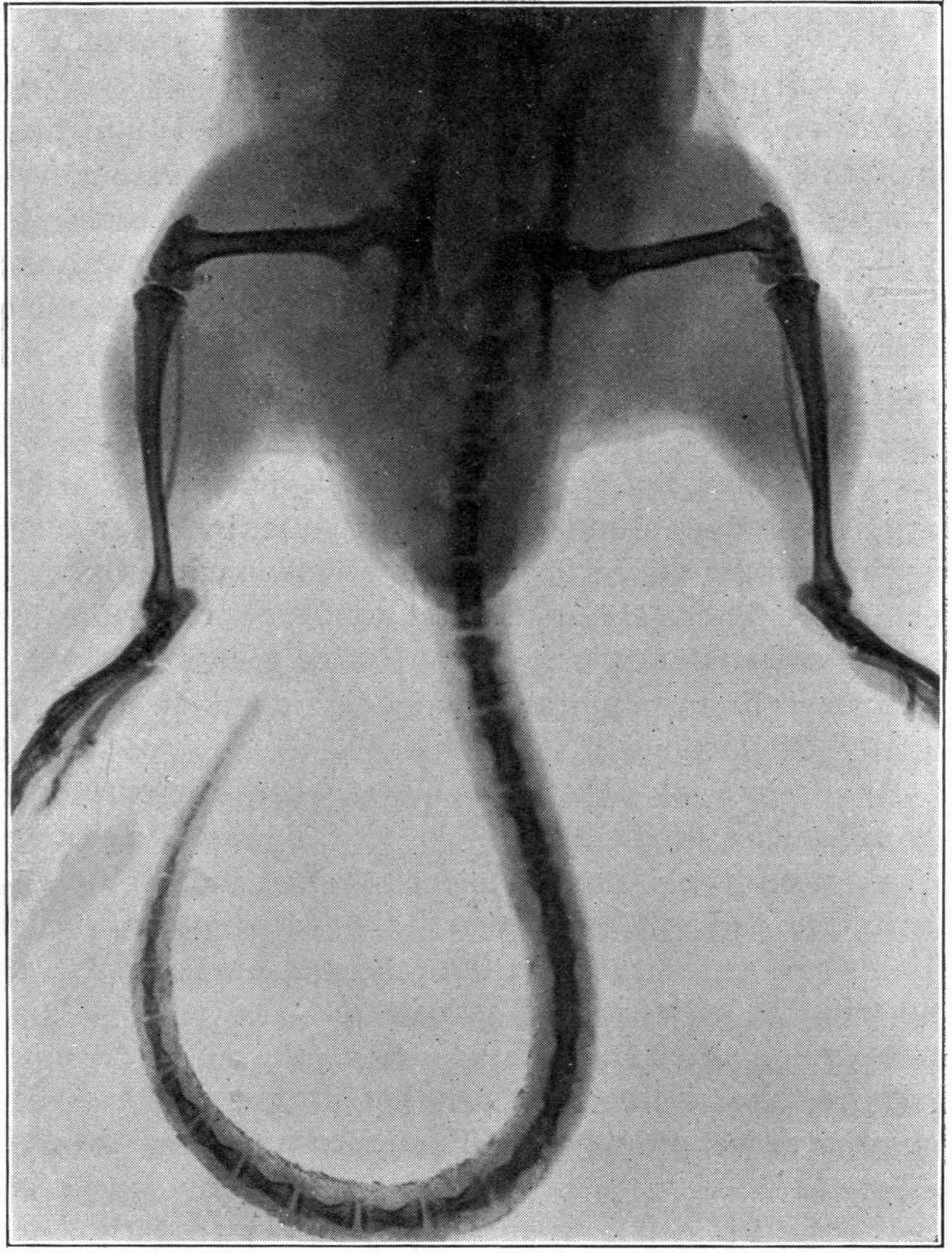
Mr. Oliver B. Shallenberger, (Mem. Amer. Inst. Elec. Eng.) whose laboratory is in Rochester, Pa., gave some important general instructions concerning the Tesla system § 51, that he employed in the production of remarkably clear sciagraphs, in conjunction with the focus tube, § 91, representing the hand at page 68, and showing a rat shown at this § 137. (Elec. World, N.Y., March 17, ’96.) Even the ligaments were clearly shown in the sciagraph of the rat, and some of them are dimly reproduced by the half tone process. As to the apparatus and operation, which are especially important, it may be stated that the current was taken from an alternator, of a frequency of 133 periods per second, and passed through a primary coil of a transformer for increasing the E. M. F. from 100 volts to from 16 to 25 thousand. The secondary current was then passed through Leyden jars and a double cascade of slightly separated brass cylinders, whereby it was changed into an oscillatory current of an extremely high frequency, which was then conducted through the primary of a second induction coil having very few turns of 138wire, and no iron core and having a ratio of 7 to 1. By this means the E. M. F. was raised to somewhere between 160,000 volts to 250,000, and was used to energize the discharge tube for the generation of X-rays. Caution should be taken, because the current coming from the first transformer, being of large quantity and very high E. M. F. is exceedingly dangerous, but the current of the second secondary has been passed through one’s body without danger, as reported by Mr. Tesla several years ago, and confirmed by Mr. Shallenberger.
138. Phosphorescent Spot Maintained Cool.—In testing the power of the X-rays in connection with the appearance of the phosphorescent spot, Tesla noticed that they were most powerful when the cathode rays caused the glass to appear as if it were in a fluid state. § 61. To prevent actual puncture, he maintained the spot cool by means of jets of cold air. It became possible thereby to use bulbs of thin glass at the location of the generation of the X-rays. § 119. He concluded from certain results that not only was glass a better material for discharge tubes than aluminum, but because, by other tests, he found that thin aluminum cast more shadow with X-rays than thicker glass. There are, of course, many other reasons, based on mechanical construction, why glass is preferable, and also why a tube with an aluminum window is not to be desired. Principally, the latter will soon leak.
139. Expulsion of Material Particles through the Walls of a Discharge Tube.—At quite a low vacuum, and after sealing off the lamp, he attached its terminal to that of the disruptive coil. After a while, the vacuum became enormously higher, as indicated by the following steps: First, a turbid and whitish light existed throughout the bulb. This was the first principal characteristic. Next, the color changed to red, and the electrode became very hot, in that case where powerful apparatus was employed. The precaution should be taken to regulate the E. M. F., to prevent destruction of the electrode. Gradually, the reddish light subsided, and white cathode rays, which had begun, grew dimmer and dimmer until invisible. At the same time, the phosphorescent spot became brighter and brighter and hotter and hotter, while the electrode cooled, until the glass adjacent thereto was uncomfortably cold to the touch. At this stage, the required degree of exhaustion was reached, and yet without any kind of a pump. He was enabled to hasten the process by alternate heating and cooling, and by the use of a small electrode. This whole phenomenon was exhibited with external electrodes as well. He acknowledged that 139instead of the disruptive coil, a static machine could be used, or, in fact, any generator or combination of devices adapted to produce a very high E. M. F.
The reduction of temperature of the electrode he attributed to its volatilization. Without actually testing the rays with a fluorescent screen or photographic plate, he could always know their presence by the relative temperatures of the phosphorescent spot and the electrode, for when the latter was at a low temperature and the former at a high temperature, X-rays were sure to be strong.
From the fact that the vacuum became higher and higher by the means stated, he was very much inclined to believe that there was an expulsion of material particles through the walls of the bulb. When these particles which were passing with very great velocities struck the sensitive photographic plate they should produce chemical action. He referred to the great velocity of projected particles within a discharge tube, pages 46 and 47, and to Lord Kelvin’s estimate upon the same, and reasoned that with very high potentials, the speed might be 100 km per second. The phenomenon indicated, he said, that the particles were projected through the wall of the tube and he entered into an elaborate discussion on this point. He referred to his own experiment of causing the rays from an electrode in the open air to pass directly through a thick glass plate. § 13. He performed an experiment also of producing a blackening upon a photographic plate apparently by the projected particles, an electrical screen being employed to prevent the formation of sparks. § 35. which as well known will cause chemical action upon the plate. No stronger proof as to the expulsion of material particles could be desired than an operation in which the eyes can see for themselves that such an action must have taken place. Usually he was troubled by the streamers (cathode rays) from the electrode suddenly breaking the glass of the discharge tube. This occurred when the spot struck was at or near the point where the same was sealed from the pump. He arranged a tube in which the streamers did not strike the sealing point, but rather the side of the tube. It was extraordinary that a visible but fine hole was made through the wall of the tube, and especially that no air rushed into the vacuum. On the other hand, the pressure of the air was overcome by something rushing out of the tube through the hole. The glass around the hole was not very hot, although if care were not taken, it would become much hotter, and soften and bulge out, also indicating a pressure within, § 27. greater than the atmospheric pressure. He maintained the 140punctured tube in this condition for some time and the rarefaction continued to increase. As to the appearances, the streamers were not only visible within the tube, but could be seen passing through the hole, but as the vacuum became higher and higher, the streamers became less and less bright. At a little higher degree of vacuum, the streamers were still visible at the heated spot, but finally disappeared.
This electrical process of evacuating varies in its rapidity according to the thinness of the glass. Here again he noted the application of his theory in that an easier passage was afforded for the ions. § 47. A few minutes of operation produced through thin glass, a vacuum from very low to very high, whereas, to obtain the same vacuum through much thicker glass over 1/2 hour was necessary. Again with a thick electrode the time required was much greater. The small hole was not always visible and it was thought that the material went through the pores. The result obtained by the following experiment tends to uphold Mr. Tesla’s emission theory.
139a. Lafay’s Experiment. Giving to X-rays the Property of Being Deflected by a Magnet by Passing Them Through a Charged Silver Leaf. Comptes Rendus, March 23, ’96 and April 7, 13, 27, and L’Ind. Elec., April and May ’96. From trans. by Louis M. Pignolet. He placed at about .5 cm. below a discharge tube, a lead screen pierced by a slit 2 mm. wide; and 0.04 m. lower, a second lead screen having a slit 5 mm. wide completely covered by an extremely thin leaf of silver. Opposite the silver leaf and exactly in the axis of the slit, was fixed a platinum wire 1.5 mm. diameter. Thus, the rays which passed successively through the two slits projected a shadow of the wire on a photographic plate below.
When the silver leaf was connected to the negative pole of the induction coil that excited the tube, the rays, which had become electrified (§ 61b, p. 47) bypassing through the leaf, were deflected by a magnetic field of about 400 L. G. S. units, whose lines of force were parallel to the slit. The direction of the deflection was determined by the same law as that of the deflection by a magnetic field of the cathode rays in the interior of a discharge tube. § 59. When the silver leaf was not connected to the coil, no deflection was produced. § 79.
To double the apparent deflection, one part of the slit was covered by a lead plate during the first half of the experiment. The lead plate was removed and placed over the other part of the slit, and the direction of the magnetic field reversed during the last half of the experiment. Thus the distance on the 141sciagraph between the two parts of the wire, was double the deflection produced by the magnetic field.
The deflection was in the same direction when the silver leaf was connected to the negative pole of a static electric machine, but was in the opposite direction when the leaf was connected to the positive pole of the machine. The test was criticised in the scientific press, and, therefore, in order to be certain that the deflections observed were not due to the combined effects of the electro-magnet which produced the magnetic field and the electric field of the charged silver leaf, the experiments were modified. To remove this uncertainty, the electrified rays were caused to enter a grounded Faraday cylinder (see figure at E. F. G. H., p. 47), through a small opening, before passing between the poles of the electro-magnet. The deviations which were recorded on a photographic plate in the box were the same as before.
Additional experiments showed that the deflections by the magnetic field took place as well when the rays were electrified, after their passage through another magnetic field, as before. Lafay continued the experiments in great detail and by many control tests, and he took accurate measurements and followed the suggestions of others. It would be well for those who have facilities to repeat these most interesting and important researches, to determine for themselves some satisfaction.
It is of interest to note that an American, Paul A. N. Winand, (Mem. Amer. Inst. Elect. Engs.), in the absence of facilities for experimenting, proposed (Elect. World, N.Y., June 6, ’96) to interpose a hollow sphere, which had high potential, in the path of X-rays, and to learn in what manner, if any, the rays are influenced. He argued that it would seem natural that, inasmuch as the rays produce a discharge, there should be a reaction of the charged surface upon the rays.
It is evident that if any one repeats these experiments, expert manipulation is required.
139b. Gouy’s Experiments. The Penetration of Gases into the Glass Walls of Discharge Tubes. Comptes Rendus, March 30, ’96. From trans. by Louis M. Pignolet. From observations with slightly different glass from four tubes, it seemed that the cathode rays cause the gases in the tubes to penetrate the glass where they remain occluded until the glass is nearly softened (after cutting off the current), by heat, whereby they are set at liberty as minute bubbles visible by the microscope, which finally partly combine and become visible to the naked eye.
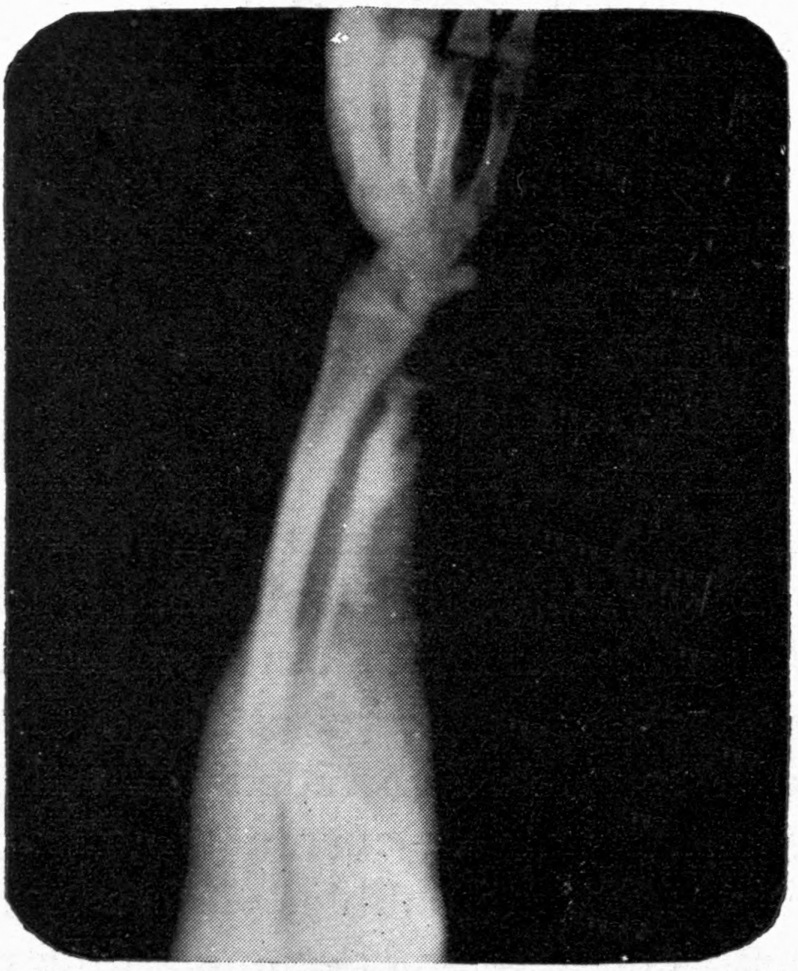
Mortification of the Ulna. § 204.
From sciagraph by Prof Miller.
143Under the same conditions, tubes which have been used for a long time exhibit numerous wrinkles, indicating a superficial modification of the glass. These may exist with or without the bubbles.
140. Discharge Tube Surrounded by a Violet Halo. By means of enormous potential and high frequency, the tube was surrounded, Tesla stated, by violet luminosity or halo. § 6. and 74. From the fact that Lenard obtained a similar appearance in front of the aluminum window, it might be reasonable to conclude that there is some close relationship between the two phenomena.
As an illustration of halo by light, may be mentioned the well known appearance so often occurring in the atmosphere concentrically with the moon, and sometimes surrounding the sun. Under favorable circumstances, (in a mist or dust in the air), a halo, at some distance from a flame or other light is faintly visible. It has generally been assumed that the reason of a halo by light is based upon the laws of reflection, or refraction or both, the bending of the rays taking place, through, or upon the surface of the particles of moisture. Others have held that particles of ice in the upper atmosphere, are the reflectors or refractors, or both. More puzzling has been the attempt to explain the novel appearance reproduced fairly well in the cut, page 140. It is here represented in print for the first time, but the photograph from which it was taken, was at various times, shown to different physicists, some of whom attributed the beautiful effect to the property of interference of light, and naming Newton’s rings as an analogous production. Prof. Anthony in an interview expressed himself as well satisfied that interference could not occur under the circumstances named. He recognized that there was a curved surface of glass which might be considered as made up of an infinite number of layers. The author introduces the matter for the purpose of consideration by others, and especially because it is so intimately connected with the subject of the vacuum tube and electricity. The details must be understood for the purpose of proper appreciation. Mr. William J. Hammer, of New York, had a photograph taken of the large Concert Hall at the Crystal Palace, Sydenham, Eng., by the light of the Edison incandescent lamps with which the Hall was illuminated. This photograph was made in 1882 during the International Electrical Exhibition held at the Crystal Palace. The picture shows a small section of the whole photograph and represents (although probably no one would judge so by looking at the picture) a festoon of pear-shaped 144incandescent electric lamps, each one hanging downward, and separated from its neighbor by between three and four feet. They were so far away from the camera that a picture of the lamps unlighted, would have represented them as mere specks. The bright circles with the bright central crosses in the centre of the dark spaces were, therefore, fully one foot in diameter, while the lamp bulbs themselves were only about two or three inches thick, as usual. Why then should there be the halos? Why should the crosses appear? And why should the black area be so large? If the electricity and vacuum have nothing to do with it, why should not the halos appear when photographs are taken of flames and other sources of light in the absence of mist and dust? In order to answer questions which will perhaps be proposed, let it be stated that there was no visible dust nor moisture in the room, the photograph being taken early in the evening and at a time when the Hall was not in use. The halos were not apparent except when reproduced by a photograph. The lamps had the usual carbon filaments hanging so that the several filaments were in different planes, and they were of 16 candle power and were connected in parallel circuit, the average E. M. F. being about 110 volts. The lamps were fed by the Edison direct current dynamos. The festoon shown, is one of a dozen or more which were suspended between the columns rising from the gallery and supporting the roof and were hung about forty feet from the floor. The hall was further illuminated by a huge electrolier pendant from the centre of the ceiling. These details were obtained from Mr. Hammer, who planned the installation.
141. Anæsthetic Properties of X-rays.—Tesla reported that he and his assistants tested the action of the rays upon the human system, and found that upon continued impact and penetration of the head by very powerful radiations, strange effects were noticed. He was sure that from this cause a tendency to sleep occurred (§ 84, at end), and the faculties were benumbed. He said that time seemed to pass quickly. The general effect was of a soothing nature, and the top of the head seemed to feel warm under the influence of the rays. Incidentally, he noticed, as he stated, “When working with highly strained bulbs, I frequently experienced a sudden and sometimes even painful shock in the eye. Such shocks may occur so often that the eye gets inflamed, and one cannot be considered cautious if he abstains from watching the bulb too closely.”
The author calls to mind the reports in the daily press that Edison also noticed that the eyes were in some way sensitive to 145the rays. The eye, it was reported, became fatigued at the time, and yet later, objects could be more easily distinguished.
In this connection, it should be remembered that there are not only cathode rays, X-rays, etc., but the electric force that Lenard spoke of in the neighborhood of the discharge tube, and in testing the effects upon the eyes, of course, the precaution should be taken to determine whether cathode rays, X-rays or the electric sparks are answerable for the peculiar effects. Roentgen reasoned, § 84, that the eyes were not sensitive, but the rays, in his case, were not strong enough to travel 40 to 60 feet, as in Tesla’s experiments, but only 2 m. (about 7 ft.).
142. Sciagraphs of Hair, Fur, Heart, Etc., by X-rays.—Tesla was probably the first to be at all successful in the representation in sciagraphs of such objects as hair and cloth and similar easily permeable objects. In the case of a rabbit, not only was the skeleton visible, but also the fur. Sciagraphs of birds exhibited the feathers fairly distinctly. The picture of a foot in a shoe not only represented the bones of the foot, and nails of the shoe, but every fold of the leather, trowsers, stockings, etc. His opinion as to the useful application of the rays was that any metal object, or bony or chalky deposit could be “infallibly detected in any part of the body.” In obtaining a sciagraph of a skull, vertebral column, and arm, even the shadows of the hair were clearly apparent. It was during such an experiment that the anæsthetic qualities were noticed. The author saw several of the above named sciagraphs. Furthermore, on the screen he believed he detected the pulsations of the heart. Elect. Rev., N.Y., May, 20, ’96.
Although we do not doubt this report concerning what Mr. Tesla saw, yet some scientific men are exceedingly dubious concerning the results obtained by other scientists, unless the same are confirmed by additional witnesses. It will certainly be of interest to such skeptics to have corroboratory evidence. In company with Prof. Anthony, Mr. Wm. J. Hammer and Mr. Price, editor of the Elect. Rev., N.Y., the author visited a laboratory at 31 West 55th street, New York City, for the purpose of beholding the pulsations of the human heart by means of an experiment performed by Mr. H. D. Hawkes, of Tarrytown, N.Y. There was nothing new about his apparatus, the admirable results being due merely to accurately proportioned electrical and mechanical details and skillful manipulation. The Tesla system was not used, but merely a large induction coil and rotary interrupter, and a direct current from the incandescent lamp circuit of 110 volts, all substantially as Roentgen himself 146employed. The sciascope was provided with the Edison calcic tungstate screen. In order to overcome the sparking between the terminals on the outside of the tube after a few minutes of use, he heated the cathode end by means of a Bunsen burner flame. § 139, near beginning. The utility consisted in the evaporation of condensed moisture upon the cool end of the discharge tube. The temporary heating always prevented, for several minutes, any sparking on the outside. After some preliminary experiments, each person, in turn, pressed the sciascope upon the breast of another, at the location of the heart, while the discharge tube was directly at the back of a young man. The ribs and spinal column were visible, and, projecting from the spine, appeared a semi-circular area, which expanded and contracted. Any one viewing such an operation, and knowing that he is looking at the movements of the heart, cannot but be impressed with weird wonder, and cannot but credit great honor, not only to Roentgen and Lenard, but to all those early workers who have gradually but surely, successfully made discovery after discovery in the department of the science of discharges, finally culminating in the most wonderful discovery of all.
The author remembers seeing in some medical paper that William J. Morton, M.D., of New York, had also witnessed the beating of the heart with the sciascope at an early date. Similar reports are occurring weekly.
§ 142a. Mr. Norton, of Boston (Elect. World., N.Y., May 23, ’96) also stated that the heart could be seen in faint outline, and also its pulsations. The lungs were very transparent. The liver being quite opaque, its rise and fall with the diaphragm was plainly followed. Others have suggested drinking an opaque (to X-rays) liquid, like salt water, and tracing its path.
143. Propagation of X-rays through Air to Distances of 60 Ft.—In Roentgen’s first experiments, the maximum distances at which the fluorescent screen was excited was about 7 ft. Tesla obtained similar action at a distance of over 40 ft. Photographic plates were found clouded if left at a distance of 60 ft. for any length of time. This trouble occurred when some plates were in the floor above and 60 ft. distant from the discharge tube. He attributed the wonderful increase largely to the employment of a single electrode discharge tube, because the same permitted the highest obtainable E. M. F. that could be desired.
144. X-rays with Poor Vacuum and High Potential.—In the course of Tesla’s experiments, he observed that the 148Crookes’ phenomena and X-rays could be produced without the high degree of vacuum usually considered necessary, § 118. but by way of compensation, the E. M. F. must be exceedingly high, and, of course, the tube and electrical apparatus substantially of those dimensions involved in Tesla’s work. One must be careful not to over-heat the discharge tube, which is likely to occur by increase of potential. He gave definite instructions for preventing the destruction of the tube by heating, by stating that it is only necessary to reduce the number of impulses, or to lengthen their duration, while at the same time raising their potential. For this purpose, it is best to have a rotary circuit interrupter in the primary instead of a vibrating make and break, for then it becomes convenient to vary the speed of the interrupter, which may be, evidently, so constructed that the duration of the impulses may also be varied, for example, by different sets of contact points arranged on the rotary interrupter, and made of different widths.
145. Detail Construction and Use of Single Electrode Discharge Tubes for X-rays. He pointed out that with two electrodes in a bulb as previously employed by nearly all experimenters, or an internal one in combination with an adjacent external one the E. M. F. applicable was necessarily greatly limited for the reason that the presence of both, or the nearness of any conducting object “had the effect of producing the practicable potential on the cathode.” Consequently he was driven, as he said, to a discharge tube having a single internal electrode, the other one being as far away as required. § 9. In view of his ingenious arrangements of the disruptive coil, and circuits, condensers and static screens for the bulb, he found it immaterial to pay attention to some other details followed by experimenters. For example, it made comparatively little difference in his results whether the electrode was a flat disk or had a concave surface.

The form of tube described by Tesla in full, will hereinafter be alluded to as exhibited in the several figures accompanying this description, and it consisted, therefore, of the long tube “b” made of very thick glass except at the end opposite the electrode “e”, where it was blown thin, p. 149. The electrode was an aluminum disk having a diameter only slightly less than that of the tube and located about one inch beyond the rather long narrow neck n, into which the leading-in wire c entered. It is important that a wrapping w be provided around this wire, both inside and outside of the tube. The sealing point was on the side of the neck. An electric screen has been referred to heretofore. It is 149lettered s, and was formed of a coating of bronze paint applied on the glass between the electrode and neck n. The screen could be made in other ways, for example, as shown at s, Fig. 2, where it consists of an annular disk behind and parallel to the electrode disk. This ring s in Fig. 2. must be placed at the right distance back of the electrode e, but just how far can only be determined by experience. The unique service of the screen was that of an automatic system for preventing the vacuum from becoming too high by use. The peculiar action was as follows, namely from time to time, a spark jumped through the wrapping w within the tube to the screen and liberated just about enough gas to maintain the vacuum at an approximately constant degree. Another way in which he was able to guard against too high a vacuum, consisted in extending the wrapping w to such a distance inside of the tube, that the same became heated sufficiently to liberate occluded gases. As to the long length of the leading-in wire within a long neck behind the 150cathode, Lenard found the same to be valuable in conjunction with a wrapping around the wire. With high potential, a spark, at a certain high degree of vacuum, formed behind the electrode, and prevented the use of very high potential, but by having the wire extend far into the tube and providing wrappers, the sparking was much less likely to occur. By proper adjustment as before intimated, Tesla could produce just about enough to compensate for the electrical increase of the vacuum. Another difficulty that presented itself in connection with high E. M. F. was the undue formation of streamers heretofore referred to, apparently issuing from the glass, and so often disabling it. He therefore immersed the discharge tube in oil as pointed out in detail hereinafter. The walls of the tube served to throw forward to the thin glass many of those rays that otherwise would have been scattered laterally. Upon comparing a long thick tube of this kind with a spherical one, the sensitive plate was acted upon by the rays in 1/4 the time with the tube. A modification consisted in surrounding a lower portion of the tube, with an outside terminal e, indicated in dotted lines in Fig. 1. In this way the discharge tube had two terminals. The greatest advantage probably in using a long tube, was that the longer it was, within the proper limits, the greater the potential which could be applied with advantages. As to the aluminum electrode, he noticed that it was superior, in comparison with one made of platinum which gave inferior results, and caused the bulb to become disabled in an inconveniently short period of time.
146. Percentage of Reflected X-rays. He performed some preliminary experiments, testing roughly as to whether any appreciable amount of radiation could be reflected or not from any given surface. Within 45 minutes he was enabled to obtain clear and sharp sciagraphs of metal objects, and the same could have been obtained only by the reflected rays, because he screened the direct rays by means of very thick copper. By a rough calculation he found that the percentage of the total amount of rays reflected was somewhere in the neighborhood of 2 per cent.
Prof. Rood, of Columbia University, N.Y., (Sci., Mar. 27, ’96.) by means of an experiment with platinum foil, § 80, concluded that the per centage was about .005, the incident angle being 45 degrees. He regarded this figure as the mere first approximation. Judging from Roentgen, § 85, Tesla, Rood and others, therefore, it seems to be established that the percentage of X-rays reflected is very small.
Prof. Mayer, of Stevens Institute, (Science, May 8, ’96,) is of 151the opinion that there is a regular or specular reflection, having witnessed some experiments obtained by Prof. Rood, of Columbia Univ., N.Y. Prof. Mayer reported that the original negatives were taken in such a way as to substantiate regular reflection, and were carefully examined by six eminent physicists at the National Acad. of Sci. at Washington, April 23, ’96, and none had the slightest doubt concerning the completeness of the demonstration. The material employed for reflecting was platinum foil. § 103a.
Difference Between Diffusion and Reflection. Judging from the experiments above related, as well as those considered in § 103a, there might at first appear to be contradictory results, reported by different authorities. Experts, it is thought will, without argument, discover the harmonious agreement, and will commend the work of scientists, who, in different parts of the world, and at about the same time, made similar experiments, which now being considered jointly, are found to agree so wonderfully closely. Upon reading the above sections and those referred to, there can be no doubt whatever but that X-rays, upon striking a body are, to a certain per cent. scattered, or thrown back, or bent from their straight course, and sent in a backward and different direction, at one angle or another. The only apparent absolute contradiction to this is that of Perrin, § 103a, near the end. But his is a case of one witness against scores, and, therefore, evidence based upon his experiments, must be counted out. The error was either due to some oversight of his own, or more probably the mistake is merely a typographical one, for often a mistake creeps in between a man’s dictation and the printed work. It is difficult to accuse Perrin of a mistake, for he is a great French authority in such matters. Assuming that no error has occured, let it be noticed that he does not pronounce non-reflection from all substances, but only from steel p. 154, l. 9, and flint, which have been so polished as to form a mirror-like surface, whereas all other experimenters, with scarcely an exception, have not employed such surfaces. The difficult point to believe is that, after six hours, no energy from the mirror could be collected. If we accept Perrin’s results it must be only in regard to those two particular materials, polished steel and flint. Another feature which is on the edge of conjecture, is that of true or specular reflection, referred to by Prof. Mayer, § 146. Many attempts have been undertaken to prove whether the rays were thrown backward on the principle of reflection as light from a mirror, or of diffusion as light from chalk. Let the student notice that the evidence is overwhelming 152in favor of the turning back of the rays to a very small per cent. upon striking any object. As to specular reflection, which means similar to the reflection of light from a polished mirror, it is practically the same as diffusion, the difference being substantially of a technical nature. This allegation is based upon the detail distinction between reflection and diffusion given by P. G. Tait, professor of natural philosophy, Univ. of Edinburgh, who states, in Encyclo. Brit., vol. 141, p. 586:—
“It is by scattered light that non-luminous objects are, in general, made visible. Contrast, for instance, the effect when a ray of sunlight in a dark room falls upon a piece of polished silver, and when it falls on a piece of chalk. Unless there be dust or scratches on the silver, you cannot see it, because no light is given from it from surrounding bodies except in one definite direction, into which (practically) the whole ray of sunlight is diverted. But the chalk sends light to all surrounding bodies, from which any part of its illuminated sides can be seen; and there is no special direction in which it sends a more powerful ray than in others. It is probable that if we could, with sufficient closeness, examine the surface of the chalk, we should find its behavior to be in the nature of reflection, but reflection due to little mirrors inclined to all conceivable aspects, and to all conceivable angles to the incident light. Thus scattering may be looked upon as ultimately due to reflection. When the sea is perfectly calm, we see it in one intolerably bright image of the sun only. But when it is continuously covered with slight ripples, the definite image is broken up, and we have a large surface of the water shining by what is virtually scattered light, though it is really made up of parts each of which is as truly reflected as it was when the surface was flat.”
146a. Reflected and Transmitted X-rays Compared.—In order to carry on a series of investigations, Mr. Tesla constructed a complete special apparatus represented in Fig. 2, p. 149, and embodied in it also an idea which he attributed to Prof. William A. Anthony, which consisted in arranging for sciagraphs to be produced by the rays transmitted through the reflecting substance as well as by the reflected rays themselves. The figure serves to show at a glance the construction and, therefore, the explanation need be but brief. It consisted of a T tube, having three openings, those at the base and side being closed by photographic plates in their opaque holders, which carried on the outside the objects o and o´ to be sciagraphed. At an angle to both plates, and centrally located, was a reflecting plate, r, which could be replaced by plates of different materials. 153At the upper opening of the plate B was a discharge tube, b, placed in a heavy Bohemian glass tube, t, to direct the scattered rays downward as much as possible from the electrode, e, to and through the thin end of the discharge tube. The objects to be sciagraphed, namely o and o´, were exact duplicates of each other. No statement could be found as to the thickness of the tested plates, r, except that they were all of equal size. The distance from the bottom of the discharge tube to the reflecting plate, r, was 13 inches, and from the latter to each photographic plate about 7 inches, so that both pencils of rays had to travel 20 inches in each instance. One hour was taken as the time of exposure. After a series of experiments with a great many different kinds of metals, they arranged themselves as to their reflecting power, in an order corresponding to Volta’s electric contact series in air. § 153. The most electro-positive metal was the best reflector, and so on. For exhaustive investigations upon the discovery of Volta, see “Experimental Researches” of Kohlrausch, Pogg. Ann., ’53, and Gerland, Pogg. Ann., ’68. The metals Tesla tested were zinc, lead, tin, copper and silver, which were, in their order, less and less reflecting, and the order is the same in the electro-positive series, zinc being the most positive, and the others less and less so, in the order named. For a complete list of the metals arranged by the Volta series, see any standard electrical text-book. He could not notice much difference between the reflecting powers of tin and lead, but he attributed this to an error in the observation.
He tried other metals, but they were either alloys or impure. Those named in the list above were the pure metals. However, he carried on experiments with sheets of many different substances, and arrived at the following table, which shows particularly the relative transmitting and reflecting powers of the various substances in the rough.
Reflecting Body |
Impression by Transmitted Rays. |
Impression by Reflected Rays. |
|---|---|---|
| Brass | Strong | Fairly good |
| Toolsteel | Barely perceptible | Very feeble |
| Zinc | None | Very strong |
| Aluminum | Very strong | None |
| Copper | None | Fairly strong but much less than zinc |
| Lead | None | Very strong but a little weaker than zinc |
| Silver | Strong, a thin plate being used | Weaker than copper |
| Tin | None | Very strong about like lead |
| Nickel | None | About like copper |
| Lead-glass | Very strong | Feeble |
| Mica | Very strong | Very strong about like lead |
| Ebonite | Strong | About like copper. |
154By comparing, as in previous experiments, the intensity of the photographic impression by reflected rays with an equivalent impression due to a direct exposure of the same bulb and at the same distance, that is, by calculations from the times of exposure under assumption that the action upon the plate was proportionate to the time, the following approximate results were obtained:
Reflecting Body |
Impression by Direct Action |
Impression by Reflected Rays. |
|---|---|---|
| Brass | 100 | 2 |
| Tool steel | 100 | 0.5 |
| Zinc | 100 | 3 |
| Aluminum | 100 | 0 |
| Copper | 100 | 2 |
| Lead | 100 | 2.5 |
| Silver | 100 | 1.75 |
| Tin | 100 | 2.5 |
| Nickel | 100 | 2 |
| Lead-glass | 100 | 1 |
| Mica | 100 | 2.5 |
| Ebonite | 100 | 2 |
He stated that while these figures can be but rough approximations, there is, nevertheless a fair probability that they are correct, in so far as the relative values of the sciagraphic impressions of the various objects by reflected rays are concerned.
In order to devise means for testing the comparative reflecting power in a more decided manner, he laid pieces of different metals side by side upon a lead plate. Consequently the reflecting surface was formed of two parts corresponding to the two metals. § 80. The vertically perpendicular partition of lead served to prevent the mingling of the rays from the two metals. Ingenious precautions were taken; as for example, so arranging matters that upon equal areas of the two plates, equal amounts of X-rays impinged. § 80. He undertook to determine the position of iron in the series by thus comparing it with copper. It was impossible to be sure which metal reflected better. The same regarding tin and lead and also in reference to magnesium and zinc. Here, a difference was noticed, namely that the magnesium was a better reflector.
He has made practical application of the power of the substances to reflect a certain per cent. of the rays by employing reflectors for the purpose of reducing the time required for exposure of the photographic plates. It admits, he stated, of the use of reflectors in combination with a whole set of discharge tubes, whereby rays which would be otherwise scattered in all directions are brought more nearly to a single direction of propagation.

From Sciagraph of Knee-joint. Straight, Front View.
By Prof. Goodspeed. Photo. Times, July, ’96.
It might be argued, that in as much as zinc would reflect only about three per cent. of the incident rays, no practical gain would 156ensue in sciagraphy by the use of a reflector. He pointed out the falsity of such an argument. In the first place, the angle employed in these tests was 45°. With greater angles, the proportion of reflected rays would be greater assuming that the law of reflection is the same as that of light. By mathematical calculation and tests, he showed that there was no doubt whatever about the advantage of using reflectors. He obtained a sciagraph, on a single plate, of the ribs, arms and shoulder, clearly represented. He stated the details as follows. “A funnel shaped zinc reflector two feet high, with an opening of five inches at the bottom and 23 inches at the top, was used in the experiment. A tube similar in every respect to those previously described, was suspended in the funnel, so that only the static screen of the tube was above the former. The exact distance from the electrode to the sensitive plate was four and one-half feet.”
147. Discharge Tube Placed in Oil.—When the E. M. F. was increased, by having the discharge tube, as usual, in open air, sparks formed behind the electrode, and within the vacuum, and endangered the life of the discharge tube. He obviated this difficulty partly by having the electrode located well within the evacuated space, so that the wire leading to it was unusually long. By excessive E. M. F., also, streamers broke out at the end of the tube. To overcome all difficulties in connection with sparking and breaking of the tube, he followed the proposition of Prof. Trowbridge, and submerged the discharge tube in oil, § 11, at end, and § 13, which was continually renewed by flowing into and out of the vessel in which the discharge tube was contained, all as shown in the accompanying figure, p. 157, “Discharge Tube Immersed in Oil.” The discharge tube, t, may be recognized by its shape, and it is located horizontally in a cylindrical tube lying sidewise upon a table. To regulate the flow of the oil, the reservoir may be raised and lowered by a bracket, s. The X-rays enter the outside atmosphere by passing first through glass, then oil, and then through a diaphragm of “pergament” forming the right hand end of the oil vessel. When the results were compared with those obtained by Roentgen in his first experiments, the rays were found so powerful that it is not surprising that Tesla was able to obtain more definitely a closer knowledge of the properties of the rays. Roentgen obtained, with his tube and a screen of barium platino cyanide, a shadow picture of the bones of the hand at a distance of less than 7 ft., while Tesla obtained a similar picture with a screen of calcic tungstate, and with 157his tube immersed in oil at a distance of 45 ft. Tesla also made sciagraphs with but a few minutes’ exposure at a distance of 40 ft., by the help of Prof. Henry’s method, i.e., with the assistance of a fluorescent powder. § 151. He referred also to Salvioni’s suggestion of a fluorescent emulsion. He attributed to Mr. E. R. Hewitt the conjecture that the sharpness of the sciagraphs might be increased by a thin aluminum sheet having parallel groves. Several experiments were made, therefore, with wire gauze, as well as with a screen formed of a mixture of fluorescent and iron-fluorescent powders. With the strong power of the rays as obtained by Tesla in combination with such adjuncts, the shadows were sharper, although the radiation, of course, was weakened by the obstruction. § 107b.
With the apparatus involving the discharge tube in oil, and with tremendously high potential, he obtained what may be called wonderful results; for with the sciascope he obtained shadow pictures of the vertebral column, outline of the hip bones, the location of the heart (and later detected its pulsations), ribs and shorter bones, and, without the least difficulty, the bones of all the limbs. More than this, a sciagraph of the skeleton of the hand was perceived through copper, iron or brass very nearly 1/4 inch thick, while glass 1/2 inch thick scarcely dimmed the fluorescence. The skull of the head of an assistant acted likewise, while at a distance of three feet from the discharge tube. The motion of the hand was detected upon the screen although 158the rays first passed through one’s body. In making observations with the screen, he advised that experimenters should surround the oil box closely, except at the end, with thick metal plates, to prevent X-rays from coming in undesired directions by reflection from different objects in the room. Obviously the shadows will be sharper.
148. Bodies Not Made Conductors by X-rays. Tesla referred to Prof. J. J. Thomson as having announced some time ago “that all bodies traversed by Roentgen radiations become conductors of electricity.” The author has witnessed other similar expressions giving credit to Thomson in this respect, but he understands that Prof. Thomson, having discovered that X-rays discharge both negatively and positively charged bodies, conjectured or drew a corallary as to the probability of the bodies therefore becoming conductors. Tesla, nevertheless, seems to have proved that the corallary does not hold. In the first place he employed the very powerful rays, and next, he let the oil be the substance traversed by the rays. Besides this, he applied a sensitive resonance test. See detail treatment of his experiments on this subject in Elect. Rev., N.Y., June 24, ’93, p. 228. In brief “a secondary not in very close inductive relation to the primary circuit, was connected to the latter and to the ground, and the vibration through the primary was so adjusted that true resonance took place. As the secondary had a considerable number of turns, very small bodies attached to the free terminal produced considerable variations of potential of the latter. Placing a tube in a box of wood filled with oil and attaching it to the terminal, I adjusted the vibration through the primary so that resonance took place without the bulb radiating Roentgen rays to any appreciable extent. I then changed the conditions so that the bulb became very active in the production of the rays.”
According to the corallary above referred to, the oil should be, with such an environment and under such subjection, a conductor of electricity, but it was not. He emphasized his satisfaction in the results by saying “the method I followed is so delicate that a mistake is almost an impossibility.”
Prof. W. C. Peckham, Elect. World, N.Y., May 30, ’96, reasoned that the oscillating electro-static action upon the outside of the tube, is concerned in the production of fluorescence, and other properties of X-rays. “These oscillations are certainly synchronous with the vibrations of the cathode rays in the tube, which in turn synchronize with the oscillation in the induction coil. If the vibrations of the tube cannot keep time with those of its coil, few or no X-rays will be given out. The cause seems to 159be similar to that of sympathetic vibrations in sound. In a word, the discharge tube is a resonator for its coil, and when the coil and tube are properly attuned, the maximum effect is obtained.
149. Appleyard’s Experiment. Non-conductors Made Conductors by Current. Proc. Phil. So., May 11, Nature, Lon., May 24, ’64, p. 93. A piece of celluloid was pressed between two metal plates serving as terminals. A galvanometer was employed to indicate the diminution of resistance by time, and it also showed that the electrification was negative. When mercury was one of the metals, the abnormal results did not occur, except to a very small extent. When the celluloid was replaced by gutta percha tissue, the electrification was normal. Many non-metals were employed, and several were lowered in resistance.
149a. Resistance Somewhat Independent of Metal Particles.—Through a mixture of conducting and non-conducting materials, like a sheet of gutta percha, having brass filings imbedded therein,—with 750 volts, no current passed, and this held true until the proportion in weight of the metal to the gutta percha was 2 to 1. Let it be remembered, also, that selenium is reduced as to resistance under the influence of light.
150. Minchin’s Experiment. Resistance Lowered by Electro-magnetic Waves. Nature, Lon., May 24, ’94, p. 93.—Referring to Appleyard’s experiment, it will be noticed that he found that mixtures of certain limited per cents. of metallic particles and insulators were exceedingly high in resistance. Prof. G. M. Minchin found that such materials became conductors under the influence of powerful electro-magnetic disturbances, and that after the current was conducted, its resistance remained greatly lowered in behalf of very weak impulses, although, before the experiment, the resistance was so high. § 14a. But after the current was interrupted by moving the terminal away from the mixture, the high resisting power returned slowly, at a rate somewhat in proportion to the hardness of the mixture. The film employed consisted of shellac or gelatine or sealing wax, while among the metals was pulverized tin. In the latter case, the resistance was reduced by the electro-magnetic waves from apparent infinity to 130 ohms, the electrodes being displaced by 1 cm.
151. Pupin and Swinton’s Experiment. Sciagraphic Plates Combined with Fluorescent Salts. The Elect., Lon., Apr. 24, ’96.—Prof. Pupin, of Columbia College (Electricity, N.Y., Feb. 12, ’96—the author saw him use it Feb. 7, ’96—), was among the first, and probably actually the first, to lessen the time of exposure by a fluorescent screen. Prof. Salvioni also worked in this direction at an early date. Prof. Swinton reported some details in the matter, and he was able to obtain a sciagraph of the bones of the hand in less than 10 seconds, with a moderately excited discharge tube, whereas, without the screen the time was two minutes. He experimented first with barium platino cyanide, but the results referred to were obtained with calcic tungstate, finely ground, and made up into paste by means of gum, and dried. He spread the same upon a celluloid sheet which was placed with the celluloid side against the photographic film. The difficulty experienced first was in the formation of spots on the negative, because some of the crystals fluoresced more than others. Such a defect, however, showed that the fluorescent salt increased the rapidity of the action upon the photographic film. The result of this experiment, as well as that of others, has sufficiently established the fact that the fluorescent screen is of great importance in connection with the art of rapid sciagraphy.
Phosphor sulphide of zinc is among those which hasten photographic action. (Chas. Henry, in Comptes Rendus, Feb. 10, ’96.) Dr. W. J. Morton employed the screen in taking the sciagraph of the thorax, p. 61. The advantageous use is also confirmed by Basilewski (Comptes Rendus, March 23, ’96. From trans. by Louis M. Pignolet).—The photographic plate was covered with a sheet of paper coated with barium and platino cyanide, so that the two prepared surfaces were in contact, and the fluorescent paper was between the object and the plate.
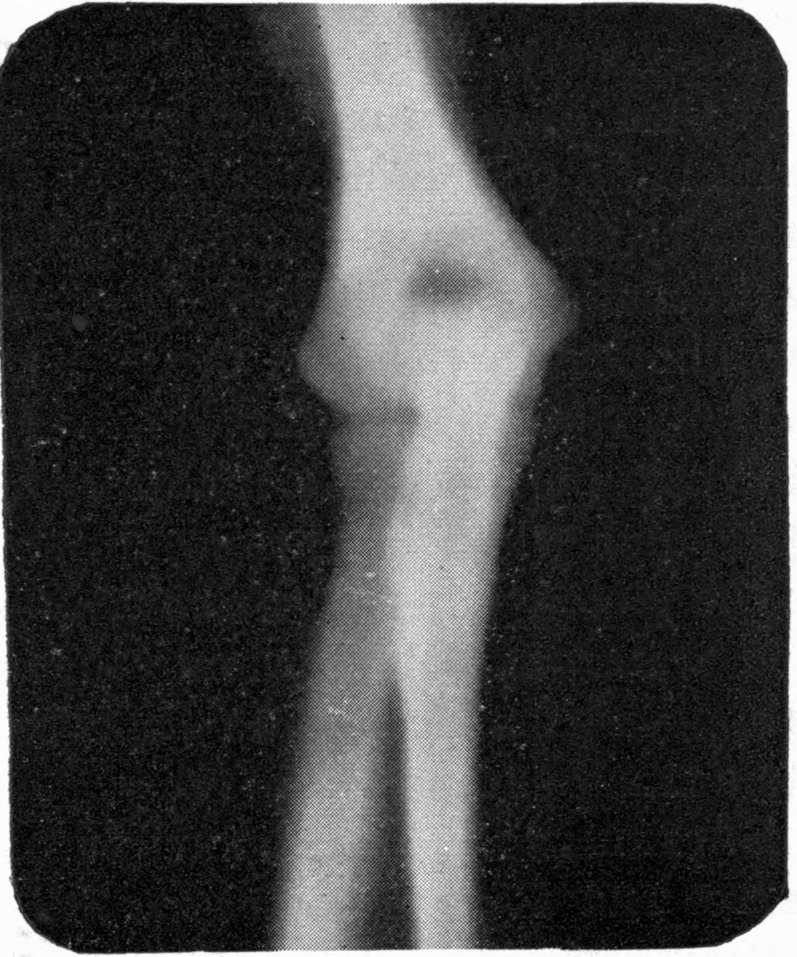
Normal Elbow. § 204.
By Prof. Miller.
J. W. Gifford, (Nature, May 21, ’96) tried a great variety of fluorescent bodies in combination with the photographic plate, and found that potassium platino cyanide was decidedly the best.
162152. Thompson’s (S. P.) Experiment. Penetrating Power of X-rays Varies with the Vacuum. Comptes Rendus. CXIII., p. 809. The Elect., Lon., April 24, ’96, p. 866. In a communication to the Académie des Sciences Prof. Sylvanus P. Thompson of the University College of Liverpool, argued that by one kind of X-rays the bones of the hand were more easily penetrated than by another kind. The two varieties were produced by different vacua. § 75 and 76. Let the vacuum be supposed to become higher and higher. At the first generation of the X-rays, the fluorescent screen showed that the bones of the hand cast very dark shadows. With increase of the vacuum, the shadows of the bones were very faint. This result is also obtained by reduction of temperature. § 152a.
152a. Bleekrode’s Experiment. Permeability at Low Temperatures Increased. Elect. Rev., Lon., June 12, ’96.—Experiments performed by him confirmed those of Edison. § 135. An experiment by Prof. Dewar strongly confirmed the results. They noticed the same peculiarity that Edison did, namely, that the shadow of the finger exhibited the flesh and bones as if they were equally transparent. Varied tests showed that the reduction of the temperature of glass increased its permeability.
153. Murray’s Experiment. Reduction of the Contact Potential of Metals by X-rays. Trans. R. So., Mar. 19, ’96. The Elect., Lon., Apr. 24, ’96, p. 857. J. R. E. Murray of the Cavendish Laboratory, at the suggestion of Prof. J. J. Thomson, carried on a long series of careful experiments, to find whether the contact potential of a pair of plates of different metals was, in any way, affected by the passage of X-rays between the plates. All the ordinary precautions were taken. The contact potential was measured by Thomson’s (Kelvin) method, see Trans. Brit. Asso., 1880. The important result obtained, was that “the air through which the rays pass, § 90, is temporarily converted into an electrolyte, § 47, and when in this condition forms a connection between the plates, which has the same properties as a drop of acidulated water, namely, it rapidly reduces the potential between the opposing surfaces of the plates to zero, and may even reverse it to a small extent.”
154. Nodon’s Experiment. Transparency of Differently Colored Media to the X-rays. Comptes Rendus, Feb. 3, ’96. From trans. by Louis M. Pignolet. The rays were passed through two openings in a thick metal diaphragm, one of which was covered by an uncolored piece of gelatine and the other by 163a piece tinted with the color to be tested. The two images were received on the same plate. The various colors tested were traversed with equal facility by the rays, § 68 and 82.
The investigation described above was made by Albert Nodon at the Laboratoire des Recherches Physiques à la Sorbonne.
This agrees with Bleunard who found that colors seemed to have no influence on the passage of the rays as water colored with various aniline colors offered no more resistance than when pure. From trans. by L. M. P. Comptes Rendus, March, ’96.
A. and L. Lumière (Comptes Rendus, Feb. 17, ’96,) observed that the X-rays act in the same manner upon colored photographic plates rendered sensitive to various regions of the spectrum. Thus, plates sensitive to red, yellow and green gave exactly the same impression, provided they had the same general sensibility to white light. While this may not be accurately so, it illustrates that materials are penetrated by X-rays independently of the laws of color.
155. Meslans. Chlorine, Iodine, Sulphur, Phosphorus, combined with Certain Compounds, Increase Opacity to the X-rays (Comptes Rendus, Feb. 10, ’96. From trans. by Louis M. Pignolet.)—Carbon in its various forms was found to be very transparent, also organic substances containing, besides carbon, only the gaseous elements hydrogen, oxygen and nitrogen; but this transparency was far from uniform. Organic substances,—ethers, acids, nitrogenized compounds (corps azotes),—were easily traversed by the rays; but the introduction of an inorganic element, as particularly, chlorine, sulphur, phosphorus, and, above all, iodine, renders them opaque. § 82. This occurs also with sulphates of the alkaloids. Iodoform, the alkaloids, picric acid, fuchsine and urea are very transparent. Metallic salts are very opaque, but this varies with the metal and the acid. Bleunard went further into details. The opacity of solutions of salts increased with the atomic weight of the metal and of the metalloid. Water was easily traversed by the rays. Solutions of bromide of potassium, chloride of antimony, bichromate of potash offered considerable opposition to the passage of the rays. Solutions of borate of soda, permanganate of potassium were easily traversed. The liquids were held in paper boxes. The experiments above related were conducted by Maurice Meslans at l’École de Pharmacie de Nancy.

From Sciagraph by Prof. Miller. § 156.
1. Real diamond.
2. Paste.
3. Glass.
4. Real diamond mounted in gold ring.
156. Buquet & Gascard’s Experiments. Action of the X-rays upon the Diamond and Its Imitations; also upon Jet. Comptes Rendus, Feb. 24, 96. From trans. by Louis M. Pignolet.—Sciagraphs taken by the X-rays showed that diamonds became 165transparent, and their shadows disappeared with long exposures; but imitation diamonds remained opaque under the same conditions. Jet was distinguished from its imitations by the same method. The diamond and jet cast clearer shadows on a fluorescent screen than their imitations.
The above tests were made by Albert Buquet and Albert Gascard, at the Cabinet de Physique de l’École des Sciences de Rouen.
The half-tone on lower half of adjacent page, 164, was taken from a sciagraph by Prof. Dayton C. Miller, of Case School of Applied Science. The differences of opacity are proved, because all were of same thickness and exposed simultaneously.
Prof. Sylvanus P. Thompson (The Elect., Lon., May 18, ’96) confirmed the above, and also found that, although the diamond is more transparent than glass, it is more opaque than block carbon or graphite.
Mineralogists are thus enabled to submit minerals to the X-ray test in making analyses.
157. Dufour’s Experiment. Inactive Discharge Tubes made Luminous by X-rays. Comptes Rendus, Feb. 24, ’96. From trans. by Mr. Pignolet.—He observed that very small and sensitive Geissler tubes phosphoresced when exposed to X-rays. § § 22, 23.
158. Beaulard’s Experiments. Non-Refraction of X-rays in a Vacuum. Comptes Rendus, Mar. 30, ’96. From trans. by Louis M. Pignolet. With prisms of ebonite, F. Beaulard held that no decided deviation could be observed within the vacuum.
159. Carpentier’s Experiment. Sciagraph Showing the Parts in Relief on a Coin. Comptes Rendus, Mar. 2, ’96. From trans. by Louis M. Pignolet. An imprint of a coin stamped upon a thin piece of well annealed aluminum by pressing the coin against the aluminum, was reproduced in a sciagraph. The raised parts of the coin were scarcely 8/100 of a millimeter high. The aluminum was 5/10 millimeter thick. This result is admirably represented by the sciagraph of an aluminum medal on page 166, taken by Prof. Dayton C. Miller, of Case School of Applied Science, Elect. World, N.Y., Mar. 21, ’96, who also made a sciagraph of a copper plate 1/4 inch thick having blow holes which appeared in the picture, but they could not be detected by light, serving to illustrate an application of the new discovery in testing the homogeneity of metals.
160. Wuillomenet’s Experiments. Transparency of the Eye to the X-rays Determined by Sciagraph of Bullet Therein. Comptes Rendus, Mar. 23, ’96. A sciagraph taken with 166an exposure of three hours showed perfectly a lead shot introduced into the vitreous media of the eye of a full grown rabbit. Therefore the opacity of the media of the eye was not absolute.
In a second series of experiments by Dr. Wuillomenet a human head was used, but the results were negative in spite of a great intensity of the rays and a long exposure, § 82.
161. Fernand Ranwez’s Experiments. Application of the X-rays to Analysis of Vegetable Matter. Comptes Rendus, Apr. 13, ’96. From trans. by Louis M. Pignolet. Sciagraphy can render valuable services in analytical researches and specially in the analysis of vegetable foods where they will show the most usual adulterations consisting of mineral substances.

Bas-relief Sciagraph, § 159, by Prof. Dayton C. Miller.
This method offers several advantages for small samples of the substances can be examined. The samples are not chemically changed. A great number of tests can be made in a short time. Lastly, the sciagraph obtained affords a permanent record.
The tests were made on samples of adulterated saffron composed of mixtures of pure saffron and saffron coated with sulphate of barium. A sciagraph taken with an exposure of three minutes showed scarcely visible imprints of the pure but strong impressions of the adulterated. See sciagraph of pen, (mineral) in holder, (vegetable), in cut at upper part of p. 164, which also shows the graphite in a wooden pencil.
167162. Errera’s Experiment. Action of the X-rays on Phycomyces. Hertz Waves and Roentgen Rays Not Identical. Comptes Rendus, March 30, ’96. From trans. by Louis M. Pignolet.—Phycomyces Nitens, when submitted to the asymmetrical action of Hertz electric waves, became curved, according to Hegler. Errera found a Phycomyces was not affected by the X-rays, thus denoting an absence of Hertz waves in the rays. Credit for the above result is due to L. Errera, from experiments made at the Laboratoire Physique and the l’Institut Solvay (Université de Bruxelles).
163. Gossart, Chevallier, Foutana and Uruanni’s Experiment, in Conjunction with J. R. Rydberg. No Mechanical Action of X-rays. Comptes Rendus, Feb. 10, Mar. 23, Apr. 13, ’96. From trans. by Louis M. Pignolet.—The former party alleged that radiations from a discharge tube caused a cessation of the rotation of the vane of the radiometer. J. A. Rydberg was not inclined to confirm such action. A. Foutana and A. Uruanni made experiments and concluded that the action was due to an electro-static force, having noticed that a Leyden jar would also produce such effect. The author made some experiments to determine the matter in reference to X-rays at a distance outside of the electro-static field. The rays would neither stop the vanes nor cause them to rotate. He made some other experiments to detect whether there was any direct mechanical power possessed by the rays; but if any, it was exceedingly feeble.
T. C. Porter made some experiments at Eton College, (Nature, June 18, ’96,) which confirmed the above results, finding that the radiometer is entirely inert to the Roentgen rays, whether they be from a properly electrically screened hot or cold tube. He distinguished between the caloric conditions, for he found that, not only will reduction of temperature vary the penetrating power of the rays, § 135 and 152a, but also will an increase of temperature.
164. Battelli’s Experiment. X-Rays Within Discharge Tube. Nuovo Cimento, Apr., ’96, p. 193; Elect. Rev., Lon., June 12, ’96.—Shortly after the announcement of the discoveries of Lenard and Roentgen, it would have been considered strange to assert that X-rays may exist inside of the discharge tube. Battelli certainly correctly infers, that inasmuch as X-rays apparently originate from the point where a material object is struck by the cathode rays, § 115, it would follow that when the said object is within the vacuum space, X-rays are propagated before they reach the glass wall of the discharge tube. It has already been noted (DeMetz, § 63a) that photographic action may be 168produced within the discharge tube. Battelli has confirmed this, not by a crude experiment, like that (failure) of some authority in England, but by a series of severe tests, leaving no doubt as to the production of photographic action. He discovered in connection with several subordinate phenomena that among the rays capable of producing a photographic impression within the discharge tube, some were deflected by a magnet and others were not, from which he concluded that X-rays may exist inside the tube, in conjunction with cathode rays, before collision with the anti-cathode. The experiment consisted in deflecting the rays by a magnet, the film being in the path that the rays would have had without a magnet. There was also a film in the path of the deflected rays. Photographic action was produced upon both. He varied the vacuum. Photographic action began at 3-10 mm., had its maximum at 1-70 mm., after which it remained constant. No photographic action was obtained upon a film placed within the tube opposite the anode, except in one case where it was exceedingly weak. Lenard continually inferred that there must be two kinds of cathode rays. § 75. Battelli has certainly sifted the two rays apart and thus proved Lenard’s conjectures. § 61b, p. 47. The Elect. Rev., Lon., pays tribute to Battelli, by offering the following opinion: “We have no hesitation in saying that Battelli, by means of interesting and ingenious experiments, has made the greatest advances in the theory of the X-rays since their discovery by Roentgen.”
In many cases the author has omitted stating, in taking sciagraphs, that the films were protected from ordinary light by opaque material. This, as a matter of course, has always been understood. Battelli also had the films wrapped in material opaque to ordinary light. Experimenters should, if possible, always employ aluminum for this purpose, because the author has always noticed that black paper or cloth permits a great deal of light to come through, even when in double thickness.
Prof. Sylvanus P. Thompson (The Electr., Lon., June 26, ’96) located a wire in a focus tube in the path of the rays between the platinum reflector and the wall of the tube. Not only was there a sciagraph of this wire produced in the sciascope, but also the Crookesian shadow of the wire on the wall of the bulb. For this experiment the exhaustion must be quite high. “At no state of exhaustion did the platinum reflector convert all the internal cathode rays into X-rays.” Both shadows were cast by the platinum reflector as the origin. More or less of the rays between the reflector and the glass were sensitive to a magnet.
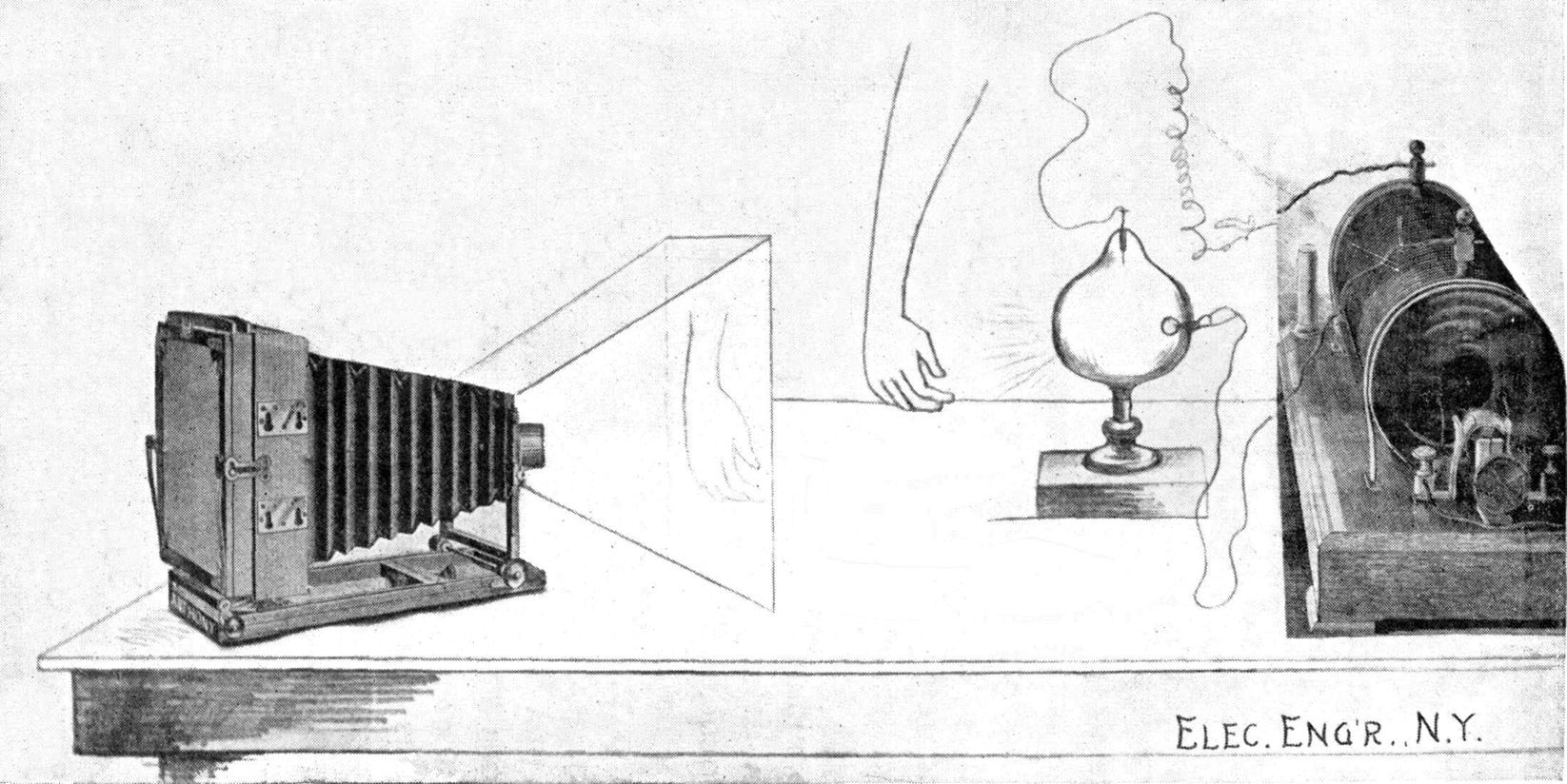
Bleyer’s Experiment. § 165.
Combined camera and sciascope at the left: and showing induction coil and discharge-tube at the right.
170165. Bleyer’s Experiment. Combined Camera and Sciascope. Elect. Eng., July 1, ’96; Royal Acad. Med. & Sur., of Naples, Italy.—As early as April 7, J. Mount Bleyer, M.D., of Naples, constructed and used the apparatus shown in the adjacent cut, p. 169. The picture is self-explanatory. Attached to an ordinary camera is a flaring sciascope, for receiving the temporary sciagraph of the hand, for example. The X-rays are converted into luminous rays by the fluorescent screen, and, therefore, the camera will serve to take a picture by means of the luminous rays from the sciagraph of the hand. The cut represents also an induction coil and a discharge tube. Soon afterwards, it was reported by an English paper that Dr. Levy, of Berlin, and others of England, had also made similar tests with success. In order to illustrate the applicability of the combination, Dr. Bleyer took many sciagraphs with the camera. He calls it the photofluoroscope, which, however, will probably not meet with favor for the name does not suggest the nature of the instrument. When two radically different devices are combined into one, it is difficult to formulate an acceptable single word, and, therefore, the instrument will probably always be called by some of the following terms: A camera with sciascopic adjustment, or combined sciascope and camera, or corresponding combinations with the word fluoroscope.
From the time that Roentgen’s discovery was announced, scientists throughout the world have made careful experiments, up to date, in all possible directions, and the time has now come when the number of experiments is rapidly decreasing, only one or two being noted now and then in the scientific press, and consisting mostly in repetition, with occasionally a slight departure, involving a radically new subordinate discovery; but in view of the great number of scientists, and of their high standing as careful experimenters, and because also of their desire to be correct in their inferences, there might seem to be little else to be investigated. Time only will tell. Before passing to the final chapters relating to other matters, a few more experiments are related in the briefest manner.
166. Prof. Sylvanus P. Thompson confirmed non-polarization, (Phil. So., June 12, ’96, and The Electr., Lon., June 26, ’96.)
Dr. John Macintyre (Nature, June 24, ’96) carried on a long series of experiments with tourmaline, and also arrived at the conclusion that polarization of X-rays is practically impossible, § 97, at end.
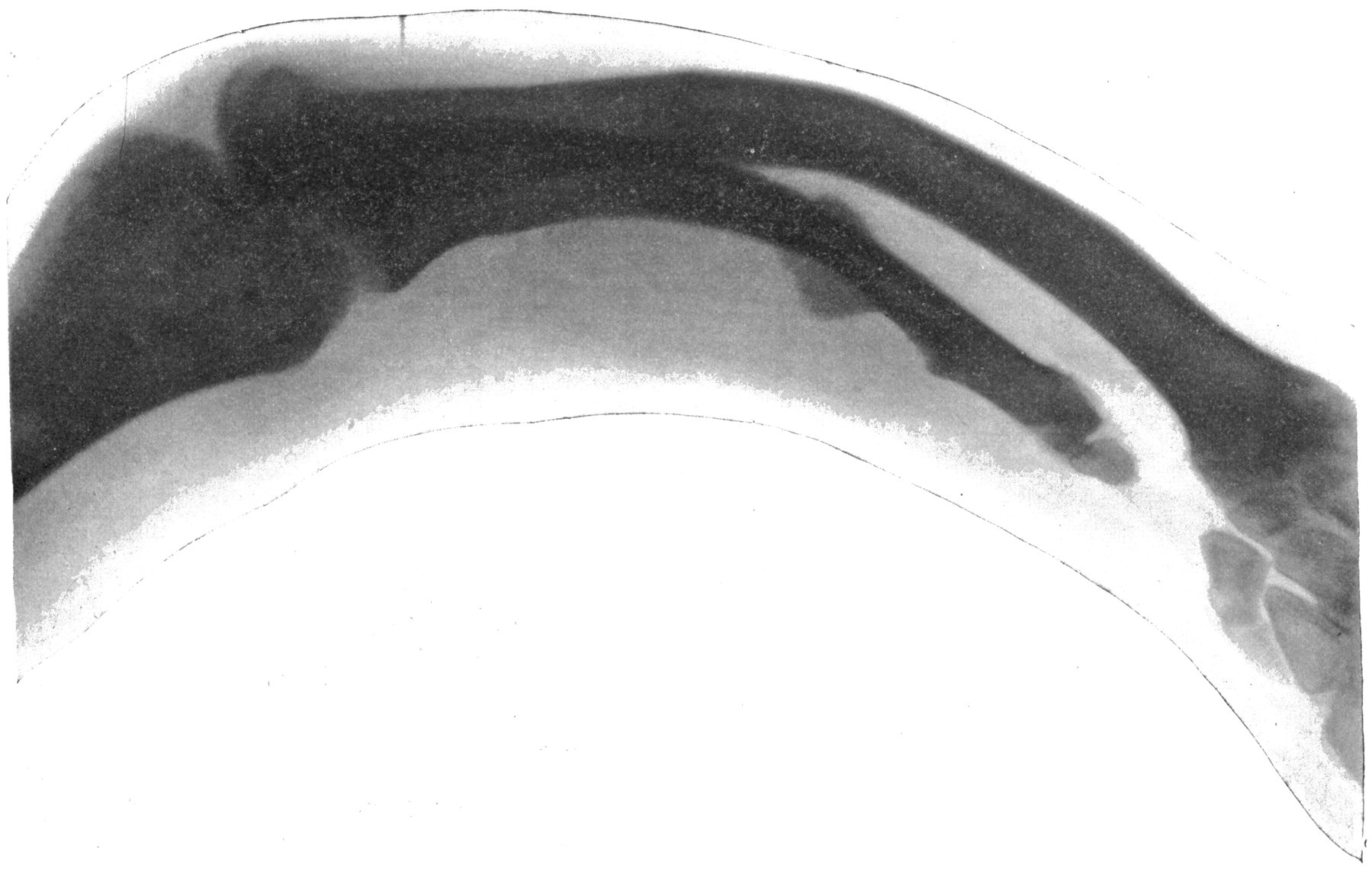
From Sciagraph by Prof. Goodspeed, showing Curvature of the Radius, due to Arrested Development of the Ulna at its Distal Epiphysis. One Bone shown through another.
167. In the same paper Prof. Thompson showed conclusively that there is a diffuse reflection of X-rays. § 81 and 103. A 172curious experiment consisted in his obtaining dust figures, § 36. by the discharge of an electrified body by X-rays. In another experiment he caused reflection of the rays from the surface of sodium located in a vacuum. The amount reflected was a minimum for normal incidence and increased at oblique incidence.
168. Prof. Oliver J. Lodge, F.R.S., reported in The Electr., Lon., June 5, ’96, further detail experiments in the line set out in § 113. He proved conclusively, as stated by the editorial in The Electrician, that a positive charge has increasing effect upon the ray-emitting power of the surface exposed to the cathodic radiation.
169. At Eton College, T. C. Porter (Nature, June 18, ’96) confirmed the experiments of others by showing that the blackened face of the thermopile connected with a very sensitive galvanometer was not influenced in any manner by X-rays.
170. Prof. William F. Magie, of Princeton, N. J., made a careful experiment in relation to diffraction. Princeton College Bulletin, May, ’96. The experiment would certainly prove that if X-rays are due to vibrations, the latter are of a different order from those occurring in light rays, for the slits exhibited light diffraction very well, but there was no evidence, by a widening of the image on the plate, that X-rays had been diffracted in the slightest degree. § 110 and 110a.
171. Prof. Haga, of Groningen University, at the suggestion of Mr. J. W. Giltay, (Nature, June 4, ’96,) made some very crucial tests, with numerous precautions, in reference to the action of X-rays upon selenium, and the results were so positive that they thought that a practical application could be made by using selenium for detecting X-rays, both qualitatively and quantitatively. In repeating the experiments, it must be borne in mind that one half hour or so is required for selenium to return to its former degree of ohmic resistance after being struck by light or heat or X-rays.
200. Hogarth’s Experiment. Needle Located by X-rays and Removed. The Lancet, Lon., Mar. 28, ’96.—Dr. Hogarth is the medical officer of the general hospital, Nottingham. A young woman was suffering with a pain in her hand near the metacarpal bone of the ring finger. A slight swelling existed. Ten weeks before, a needle had entered the palm while washing the floor. It had entered at the base of the fifth metacarpal bone. Chloroform had been given and an incision made, but no needle found and its presence doubted. A sciagraph was taken and the needle was accurately located and the next day removed.
201. Savary’s Experiment. Needle Located by Sciascope and Removed. The Lancet, Mar. 28, ’96.—Dr. Savary located a needle by a sciascope although efforts by all other methods had failed. A line was drawn between two points intersecting the needle at right angles. About half an inch below the surface of the skin of the wrist the blade of the scalpel impinged upon the needle, which was removed without difficulty.
202. Renton & Somerville’s Experiment. Diagnosis. The Lancet, Lon., Apr. 4, ’96.—A writer for the Lancet reported that Drs. Renton and Somerville made a diagnosis with the assistance of the screen. In one, the suspected case of unreduced dislocation of the phalanx, they saw that the parts were in the proper position. He showed to medical men an old fracture of the forearm where the fragments of the bones were distinct as to the shadows.
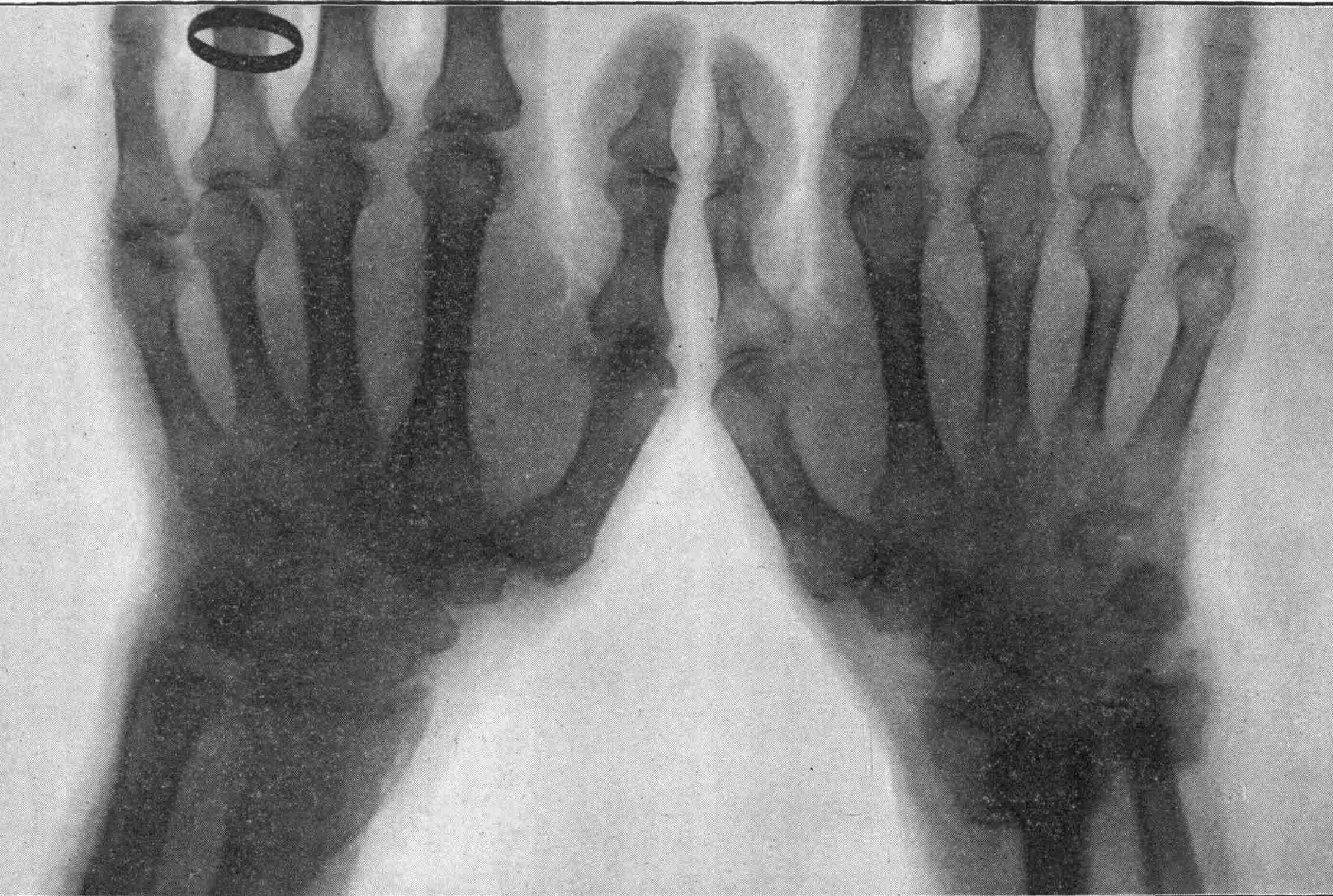
From Sciagraph of “Colles’ Fracture” in the Right Wrist, by a Fall on the Sidewalk. § 207.
By William J. Morton, M.D.
203. Miller’s Experiments. Location of Bullets. Elect. World, Mar. 21, ’96.—Bullets were clearly located in the hands of two different men by Prof. Dayton C. Miller, of the Case School of Applied Science. In one, the bullet had been lodged for 14 years and had always been thought to lie between the bones of the forearm, but two sciagraphs from different directions located the ball at the base of the little finger. By means 175of five sciagraphs from different directions, the ball in the other hand was located at the base of the thumb.
204. Injuries by Accident and Miscellaneous Cases. The Integral, Cleveland, Ohio, ’96.—Many fingers and hands were examined by Prof. Miller that had been injured by planing machines, cog-wheels, base balls, pistols, etc., and in each case the nature of the injuries was determined. Several cases of fractured arms were studied—some through splints and bandages. Some sciagraphs indicated that the ends of the broken bones had not been placed in apposition. Subsequently, an operation was performed to remedy the setting. In one case, he sciagraphed the arm from which a piece of the ulna had been removed five years previously. The necrosis had increased. Two sciagraphs at right angles to each other clearly exhibited the nature of the disease. The permanent set of the toes by wearing pointed shoes was clearly exhibited (p. 30.) The figure on page 147 is the side view of a foot in a laced shoe. The outlines of the bones can be traced, also the eyelets and the pegs in the heel, while the uppers scarcely appear. In Fig. 1 (introduction) is shown a head, only the skull being clearly reproduced. In the negative, the teeth appear and places whence the teeth have been extracted, also the jaw bones, nasal cavities and the ragged junction of the bones and cartilage. The varying thickness is represented in the cut, at the temples and ears. Fig. 2 (introduction) shows that a broken bone was badly set, the ends overlapping each other instead of meeting end to end. A sciagraph of an elbow is shown on p. 161. The flesh is scarcely visible. Fig. 3 (introduction) is a picture which reproduced the mere indication of the spine and ribs. In the original negative the collar bones, pelvis, clavicles, buckle of clothing and location of the heart and stomach were faintly outlined. Fig. 4 (introduction) is a representation of the knee of a boy 15 years old, in knickerbockers, showing the buttons clearly, and dimly a 32 caliber bullet which is imbedded in the end of the femur.
204a. Necrosis. Mortification of the ulna is represented on p. 142. Necrosis of the bone corresponds to gangrene of the soft parts; life is extinct.
205. Morton’s Experiment. Diagnosis. Elect. Eng., N.Y., June 17, ’96. Lect. before Odontological So., N.Y., Apr. 24, ’96; repeated in Dental Cosmos, June, ’96.—Dr. William J. Morton, of New York, made several important examinations of the human system by the use of X-rays.
In regard to application in dentistry, he stated:—“Each errant fang is distinctly placed, however deeply imbedded 176within its alveolar socket; teeth before their eruption stand forth in plain view; an unsuspected exostosis is revealed; a pocket of necrosis, of suppuration, or of tuberculosis is revealed in its exact outlines; the extent and area and location of metallic fillings are sharply delineated, whether above or below the alveolar line. Most interesting is the fact that the pulp-chamber is beautifully outlined, and that erosions and enlargements may be readily detected.”
206. The author saw one of Dr. Morton’s original photographed sciagraphs of the thorax, 15 inches by 11 inches, not at all creditably reproduced at page 161. In the original, to the surgeon’s eye: “The acromion and coracoid processes of the shoulder blade are clearly shown in their relations to the head of the humerus, or arm bone, and also the end of the clavicle, or collar bone, is shown in its relations to the shoulder joint. We have, in short, an inner inspection in a living person of this rather complicated joint, the shoulder, and there can be no doubt that in defined pictures of this nature even very slight deformities and diseases would be detected. It is noticeable that the front portions of the ribs are not shown, only the posterior portions lying nearest to the sensitized plate appear; also the breastbone was sufficiently dense to almost entirely obstruct the X-rays. A collar button at the back of the neck is taken through the backbone. In some of my negatives the dark outline of the heart and liver is shown as well as the outlines of tumors in the brain; but this is evidently for purposes of demonstrating the location of organs, an over-exposure, and does not, therefore, indicate the outlines of the heart.”
The time of exposure was reduced by the use of a fluorescent screen in conjunction with the photographic plate.
207. A woman was troubled with a stiffened wrist. Dr. Morton took a single sciagraph of both wrists side by side as shown at page 174, (the photographic print being presented for this book by E. B. Meyrowitz, 104 East 23d Street, N.Y.) The injured wrist in the picture exhibited the Colles’ Fracture—the ulna and radius bones being telescoped into their fractured ends by a fall upon the sidewalk a year before. By knowing the cause, the manner of cure became evident, and, accordingly, the patient is expected to bend the wrist backward and forward and laterally several times a day.
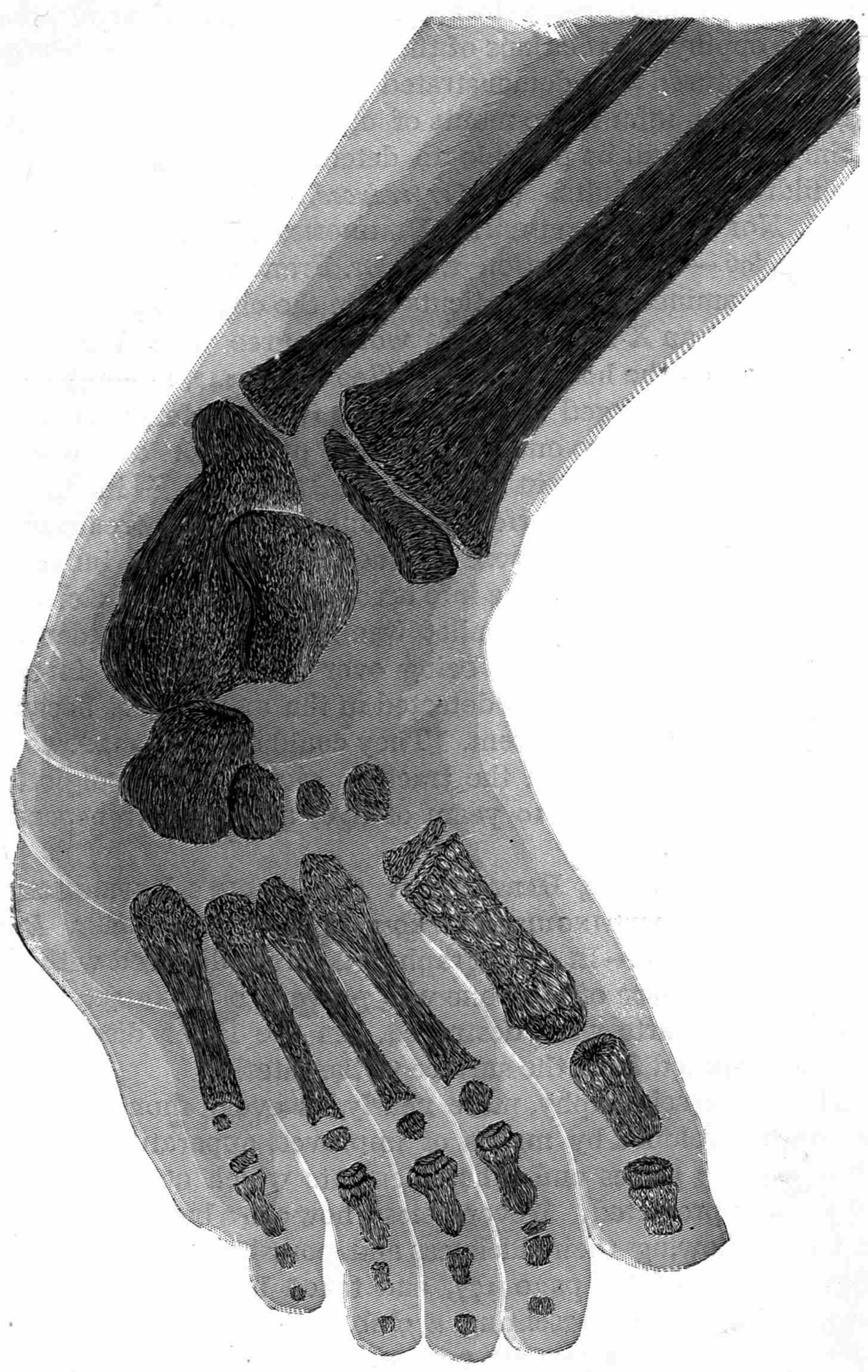
From sciagraph of club foot of child by Prof. Goodspeed. Copyright, ’96, by William Beverley Harison, Pub. of X-ray pictures, New York. This linograph (woodcut), engraved and donated by Stephen J. Cox, Downing Building, 108 Fulton St., New York, affords an exact likeness of the sciagraph,—well-nigh impossible by an untouched half-tone.
Dr. Morton, in a lecture before the Medical Society of the County of New York, to be printed in the Medical Record, related that another promising field of research and application is in the detection of calcareous infiltrations involving, for instance, 178the arteries, or occurring in the lungs and other tissues. Calculi in kidneys, in the bladder, in the salivary ducts have already been successfully located. The stages of ossification, and the epiphyseal relations of the osseous structure in children may be pictured as is demonstrated in the picture of the entire skeleton of an infant five months of age. The sciagraph shows plainly that it will be possible to detect spinal diseases, either in children or in adults. (Not reproduced.)
208. Norton’s Experiment. Diagnosis. Elect. World, N.Y., May 23, ’96.—In conjunction with Dr. Francis H. Williams, Dr. Norton examined several patients from the city hospital to determine how an X-ray diagnosis would agree with that previously made by the hospital staff. (See also § 142, at end.) The outline of an enlarged liver, 7 inches in diameter, was easily distinguished, the two outlines, one by percussion and one by X-rays, agreeing better in favor of the latter by 1/2 inch. An enlarged spleen was perfectly outlined. The tuberculosis of one lung caused it to be more opaque than the sound lung. It was found necessary to take into account the seams of clothing, buttons, buckles, etc. A bullet was found exactly under the spot which they marked as being over the bullet. A foreign metallic body can be easily detected in the œsophagus, because the latter is quite transparent. They could see the shadows of the cartilaginous rings in the trachea, glottis, and epiglottis. Younger persons, up to 10 years of age, are more transparent than older.
209. Lannelongue, Barthelemy and Oudin’s Experiments. Osteomyelitis Distinguished from Periostitis. Elec. Rev., Lon., Feb. 14, ’96.—In a sciagraph of a person diseased with the former, the surface of the bone was proved to be intact, while the internal parts were destroyed. In the latter disease the changes proceed from the surface to the interior.
The art of sciagraphy, more nearly, as every month passes, becomes developed by means of improved apparatus, screens, photographic plates and other elements which at present are only dimly predicted. Nevertheless, how can a better sciagraph of bones, showing their thickness and porosity, be desired than that reproduced on page 177, and taken by Prof. Arthur W. Goodspeed, and representing a club foot of a child? In the race to excel in this new art, no one, to the author’s knowledge, has surpassed Prof. Goodspeed, of the University of Penn., considered jointly from the standpoints of priority, superiority, quantity and variety. Dr. Keen, L.L.D., Professor in the Jefferson Medical College, of Philadelphia, stated (Inter. Nat. Med. Mag., 179June, ’96) that Prof. Goodspeed “has far eclipsed all others in these most beautifully clear sciagraphs.”
210. A book could be filled with the numerous cases of diagnosis by X-rays showing the utility. In closing this chapter, let it suffice to mention some of the sources of literature relating to this subject directly or indirectly: location of shot (by Dr. Ashhurst, Phila.) in lady’s wrist, not located by other means. Dr. Packard’s case of acromegaly; Dr. Muller’s (Germantown) location of needle in boy’s foot; cause of pain not before known; needle subsequently removed; a perfect thorax, or trunk, by Prof. Arthur W. Goodspeed, University of Pennsylvania; Thomas G. Morton’s (M. D. Pres. Acad. Surg., Phila.) application to painful affection of the foot, called metatarsaligia. All of the above noticed in Inter. Med. Mag., June, 1896. Case of a burned hand with anchylosis of the fingers, by W. W. Keen, M.D., L.L.D. Bacteria not killed by X-rays. Normal and abnormal phalanx distinguished. Fracture and dislocation sometimes differentiated by X-rays. Amer. Jour. Med. Sci., Mar., ’96.
Before attempting to discuss the facts now known in regard to the Roentgen phenomena, it is well to review briefly the known ways in which radiant energy may be transmitted.
By radiant energy is, of course, meant energy proceeding outward from a source and producing effects at some distant point. There are two well understood ways in which energy may be transmitted,—first, by an actual transfer to the distant point of matter to which the energy has been imparted from the source, as in the flight of a common ball, a bullet, or a charge of shot. In this mode of transmission, it is evident that the flying particles, assuming that they are subject to no forces on the way, will move in straight lines from the source to the distant point. They constitute real rays, diverging from the source; an obstacle in their path, would, if the radiations proceeded from a point, cast a shadow with sharply defined edges.
Second,—by a transfer of the energy from part to part of an intervening medium, each part as it receives the energy, transmitting it at once to the parts around it, no part undergoing more than a slight displacement from its normal position. This mode of transmission constitutes wave motion. The source imparts its energy to the particles of the medium near it. Each of those particles transfers its energy to the particles all around it. Each of these particles in turn transfers its energy to the particles around it, and so on through the medium. It is plain that there are here no such things as genuine rays. As the energy is transferred from particle to particle, each in turn becomes a centre of disturbance transmitting its motion in all directions. It is only because the movements transmitted from different points annul one another except along certain lines, that we have apparent straight lines of transmission, and, therefore, fairly sharp shadows. But shadows produced by wave transmissions are never absolutely sharp. The wave movement is always propagated to some extent within the boundary of the 181geometrical shadow, less as the wave lengths are shorter. With sound waves whose lengths are measured in inches or feet, the penetration into the shadow is considerable. With light waves 1/37000 to 1/70000 of an inch in length, the penetration into the shadow is very small and requires specially arranged apparatus to show that it exists.
This penetration into the geometrical shadow is characteristic of energy propagated by wave motion, and if the fact of such penetration can be demonstrated, it is conclusive proof of propagation by waves.
Another characteristic of wave motion is found in the phenomena of interference. This is the mutual effect of two wave systems, which, when meeting at a given point, may strengthen or annul each other according to the conditions under which they meet. Either of those characteristics should enable us to distinguish between propagation by wave motion and by projected particles. But when wave lengths are very short and radiations feeble, the tests are not easy to apply.
Again, a wave is in general propagated with different velocities in different media. This causes a deflection or deformation of the wave as it passes from one medium into another, and results in refraction, as in the cases of light and sound. Absence of refraction would be strong though not conclusive evidence against a wave theory of propagation.
In wave propagation, each particle of the medium suffers a small displacement from its equilibrium position and performs a periodic motion about that position. This displacement may be in the line of propagation—longitudinal vibration—or it may be in a plane at right angles to that line—transverse vibration. All the phenomena mentioned above, diffraction, interference, refraction, and also reflection, belong equally to either mode of wave propagation. Other phenomena must be made use of to distinguish between these.
When the vibrations are transverse they may all be brought into one plane through the line of propagation. They may be polarized, when the ray will present different phenomena upon different sides. When the vibrations are longitudinal, no such phenomena can be produced. Polarization, then, serves to distinguish between longitudinal and transverse vibrations.
Now let us consider briefly the Roentgen ray phenomena that bear upon the question of the nature of the propagation.

From Sciagraph of Normal Elbow-joint; Straight, in Position of Supination.
By A. W. Goodspeed. Phot. Times, July, ’96.
Copyright, 1896, by William Beverley Harrison, Publisher of “X-ray” Pictures, New York.
It seems to be settled beyond question that the origin of the Roentgen rays is the fluorescent spot in the discharge tube. § § 107, 108, 111. The evidence seems overwhelming that within 183the tube, the phenomena are the result of streams of electrified particles of the residual matter, shot off from the cathode in straight lines, perpendicular to its surface. § 57. This was Crookes’ original theory, § 53, near centre, and it seems to have stood well the test of scientific criticism. These flying particles falling upon anything in their path, give rise to X-rays. It is preferable, but not essential, that the bombarded surface should be connected electrically with the anode. § § 113, and 116. The best results are obtained by using a concave cathode, and placing at its centre the surface which is to receive the bombardment, thereby concentrating the effect upon a small area.
Nearly all experimenters agree in locating the origin of the X-rays at this bombarded spot. The energy here undergoes a transformation, and the X-rays represent one of the forms of energy developed.
What are the characteristics of this particular form of radiant energy?
It causes certain salts to fluoresce, § § 66, 84, and 132, and it affects the photographic plate. § § 70 and 84. In these respects, it is like the short wave length radiations from a luminous source. It is, however, totally unlike these in its power of penetrating numerous substances entirely opaque to light, such as wood, paper, hard rubber, flesh, etc. In passing through hard rubber and some other opaque insulators, X-rays are like the long wave length radiations from heated bodies, but X-rays penetrate many substances that are opaque to these long wave length radiations, and they are especially distinguished from all forms of radiant energy previously recognized, in their relative penetrating power for flesh and bones which makes it possible to obtain the remarkable shadow pictures which have become within three or four months, so familiar to all the world.
But these phenomena, although they serve to distinguish the X-rays from all other forms of radiant energy, do not furnish any clew to the nature of the X-rays themselves.
In attempting to formulate a theory of X-rays, the idea that first naturally presents itself is that they are due to some form of wave motion.

From Sciagraph of Knee-joint, Straight, Side View, showing Patella, or Knee-cap.
By Prof. Goodspeed. Phot. Times, July, ’96.
The characteristics of wave motion are diffraction and interference phenomena. So far, no positive evidence of diffraction, § 110, nor interference, § 89, have been recognized, although experiments, have been tried that would have shown plainly, diffraction phenomena, had light been used in place of the Roentgen radiations. § 170. We must, therefore, conclude, either that the Roentgen radiations in the experiments were 185too feeble to produce a record of the diffraction effects, or, that they are not due to wave motion at all, unless of a wave length very small even when compared with waves of light. The absence of refraction is also opposed to any wave theory of the Roentgen radiations, for it is difficult to believe that waves of any kind could travel with the same velocity through all media, which they must do if they suffer no deviation. § 86.
The next supposition naturally is, that the phenomena are due to streams of particles. It has been suggested that the rays may be streams of material particles, but this theory cannot be maintained in view of the fact that the rays proceed, without hindrance, through the highest vacuum. § § 72b and 133, near end. Neither is it consistent with the high velocity of propagation. Molecules of gas could not be propelled through air with any such velocity or to any such distance as X-rays are propagated. Tesla has claimed § 139, that the residual gases are driven out through the glass of the vacuum bulb by the high potential that he employs. This has not been confirmed by other experimenters. It has been observed that the vacuum may be greatly improved by working the bulb, § 121, that is, sending the discharge through it, but experimenters generally have found that heating the bulb impairs the vacuum and restores the original condition. The gases, were, therefore, occluded during the electrical discharge, to be again set free by heating the bulb. § 139b. The rays may be ether streams, perhaps in the form of moving vortices, but of such streams we have no independent knowledge, and can only determine by mathematical analysis, what their characteristics should be. They would not suffer refraction, and would not produce interference nor diffraction phenomena. Whether they would do what the X-rays do, go through the flesh and not through bone, through wood and not through metal, excite fluorescence, or affect the photographic plate, cannot be said. There is evidence that there are at least two kinds of X-rays, § 152, differing in penetrating power, though perhaps not differing in other respects.
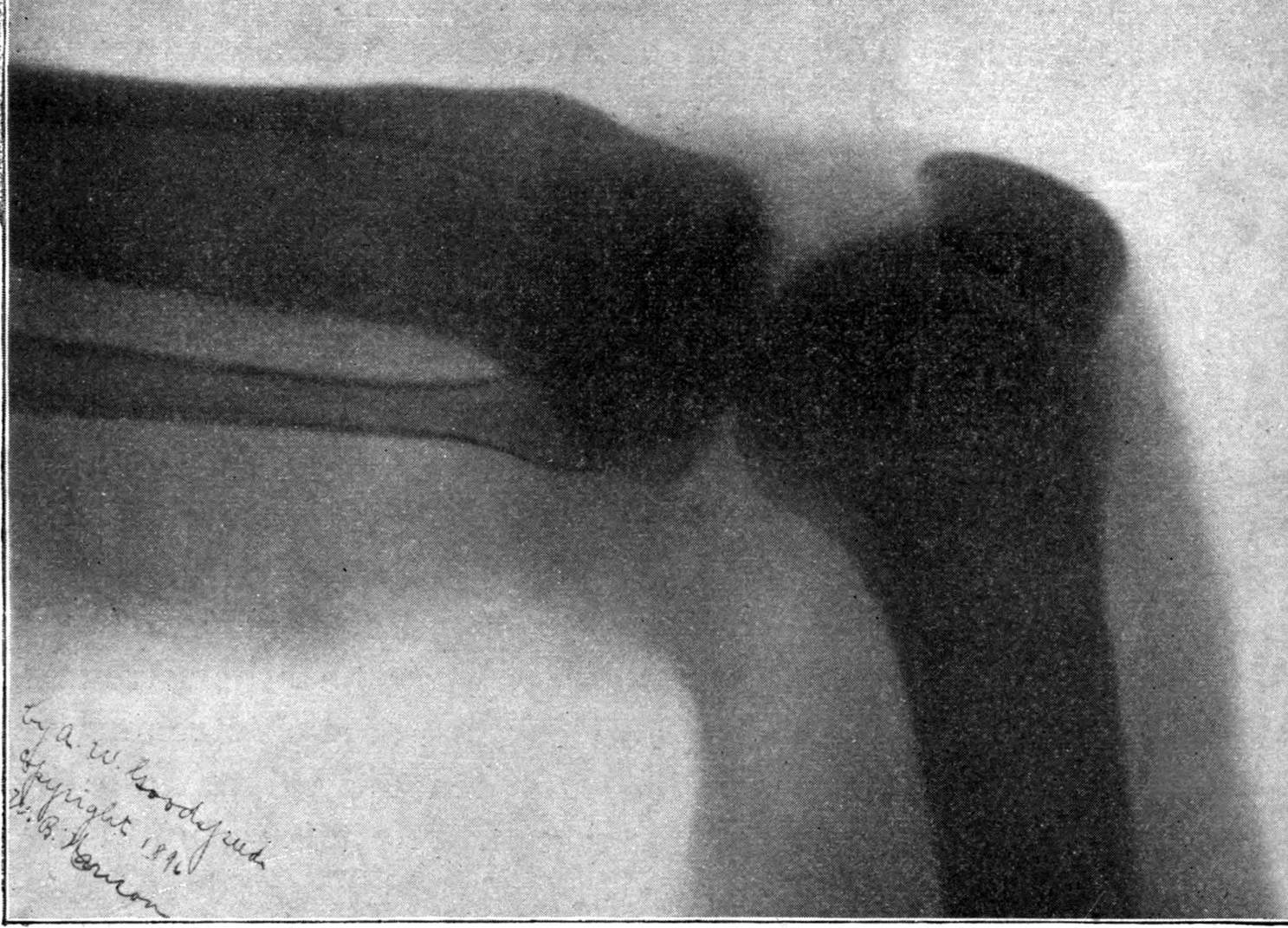
From Sciagraph of Normal Knee-joint, Flexed.
Phot. Times, July, 96.
Copyright, 1896, by William Beverley Harison, Publisher of “X-ray” Pictures, New York.
X-rays have their origin only in electrical discharges in high vacua. They are absent from sun-light and from light of the electric arc, and other sources of artificial illumination, § 136. Proceeding from the bombarded spot, they are not deflected by a magnet, except in an evacuated observing tube, as proved by Lenard, § 72a, and show no evidence of carrying an electric charge like cathode rays, § 61b, p. 47. On the contrary, they will discharge either a negatively or positively charged body in 187their path. The evidence seems conclusive (Chap. VIII.) that the ultra-violet rays from an illuminating source also discharge charged conductors. In this respect, therefore, there is a similarity between the X-rays and ultra-violet light.
The action of the waves of light upon a cell formed of selenium lowers the resistance of the latter and herein is circumstantial evidence at least, concerning the similarity of the properties of X-rays and light, because the former are also found to increase the conducting power of selenium. § 171.
The experiments of Roentgen, § 90, seem to show that the discharging effect of X-rays is due to the air through which the rays have passed.
It is certain that the discharge of electrified bodies by light occurs more generally for negatively than for positively charged bodies, § § 99B, 99I, and 99S, that it depends upon the nature, § 97b, and density, § 97a, of the gas surrounding the body, and also upon the material of the charged body itself. § 98. The discharge would, therefore, seem to be connected with a chemical action, § 153, near end, which is promoted by the rays. This seems all the more probable, since it was found, § 98, that the more electro-positive the metal, the longer the wave length that would influence the discharge. In this connection, it is well to note that Tesla found, § 146a, that in their power of reflecting (or diffusing X-rays), the different metals stand in the same order as in the electric contact series in air, the most electro-positive being the best reflectors. It would be interesting to know whether connecting the reflecting plate to earth, would, in any way, vary its reflecting power.
The X-rays seem to discharge some bodies, when positively charged, and other bodies when negatively charged. They will also give to some bodies a positive, and to others a negative charge (§ 90c). Is the order here also that of the electrical contact series in air? Are not all the phenomena of electrical charge and discharge, of reflection or diffusion, and of X-rays, connected with chemical action, as the apparent difference of potential, due to contact, undoubtedly is? § 153.
An experiment by La Fay (§ 139a) seems to show that X-rays, in air, after passing through a charged silver leaf, acquire the property of being deflected by a magnet, as are the cathode rays inside the generating or exhausted observing tube, § 72a. If this is confirmed, it would go far to support the theory that these rays are streams of something.
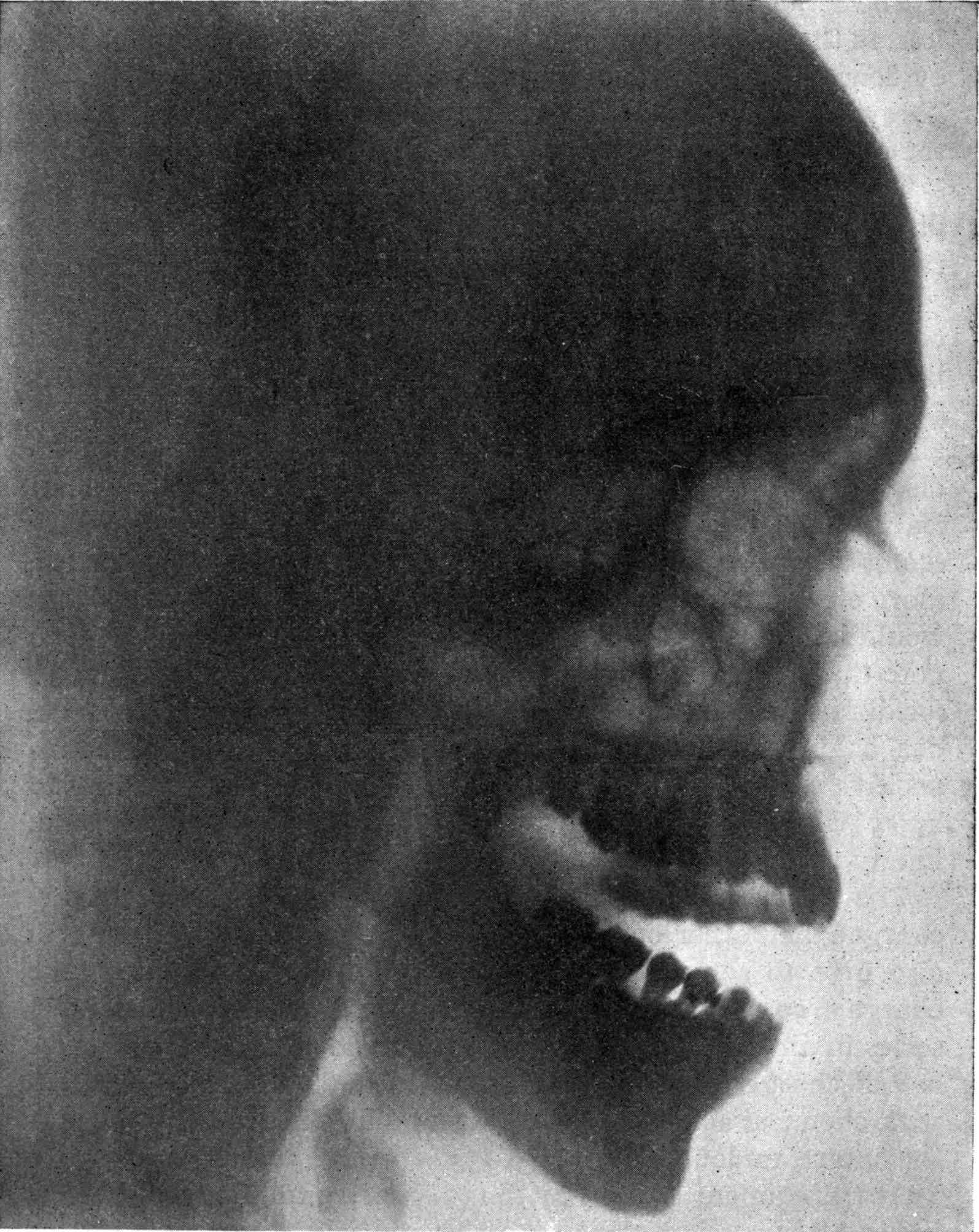
From Sciagraph of Head by Prof. Goodspeed. Nasal Bones appear like Eyelashes.
Inter. Med. Mag., June, ’96.
The cervical vertebræ are distinguishable in the original, but barely so in the half-tone. Fillings are located.
The burden of proof, up to the present, seems to be against any wave theory of the X-rays, for, although they are like the 189ultra-violet rays in producing fluorescence and in affecting the photographic plate, and have some points of similarity to these rays in their effect upon charged bodies, the X-rays are totally unlike the ultra-violet, in respect to diffraction and interference phenomena. In fact, the absence of such phenomena, if they are really absent, is conclusive proof that the X-rays cannot be wave motions, unless of a wave length extremely short even as compared to waves of light.
Since writing the above, I have seen an account of experiments in relation to diffraction of X-rays, presented to the French Academy by MM. L. Calmette and G. T. Huillier, in which the authors claim to have obtained evidence that diffraction occurs. The following translation of MM. Calmette and Huillier’s paper is taken from the Electrical Engineer, N.Y., for July 22, 1896.
“We have the honor of submitting to the Academy some photographic proofs obtained with the Röntgen rays by means of the following arrangement.”
“Very near the Crookes tube there is a screen “E” (diagram omitted), of brass, perforated by a slit, the width of which has rarely reached a half mm. A second metal screen, E´, is formed of a plate provided with two slits or pierced with a window in which is fixed a metal rod of 1 mm. in diameter. This screen is placed at the distance, a, behind the former. Lastly, a photographic plate, enfolded in two leaves of black paper, is placed at the distance, b, behind the second screen, E´.”
“The following table indicates, for each proof, what is the screen E´ used, and the value of a and b + a:
| No. | a Cm. |
b + a Cm. |
|
|---|---|---|---|
| 1. | Rod of 1 mm. in diameter | 5 | 19.5 |
| 3. | Rod of 1 mm. in diameter | 5.5 | 20 |
| 5. | Rod of 1 mm. in diameter | 8.9 | 30 |
| 7. | Two narrow slits, separated by a cylindrical rod of 1 mm. in diameter | ? | ? |
“On the proofs 1, 3, 5 the shadow thrown by the metallic rod is bordered on each side by a light band which shows a maximum of intensity. Within this shade we observe a zone less dark, which seems to indicate that the Röntgen rays penetrate into the geometrical shadow. Lastly, in proofs 3 and 5 we see, in like manner, a maximum of intensity along the margins of the window in which the rod is placed.”
“In the proof No. 7 we perceive, in the middle of the two 190white bands, a fine dark ray, while in the shadow of the rod which separates the two slits there is seen a light ray.”
“If we compare these results with those obtained with light in the same conditions, the slit being relatively wide and the intensity weak, it seems difficult not to ascribe them to the diffraction of the Röntgen rays.”
“The proofs obtained in these experiments—which we propose to continue—are not yet so distinct that we can measure the wave length with any precision. But we are still led to believe that this wave length is greater than that of the luminous rays.—Comptes Rendus.” Of course, if diffraction phenomena can be demonstrated, the question as to the radiations being wave propagations, is settled, though the question whether the vibrations are longitudinal or transverse, is still open.
Before accepting any stream or vortex motion theory, we need to know more about the X-ray phenomena, and more about stream and vortex motion.
End of the Project Gutenberg EBook of Roentgen Rays and Phenomena of the
Anode and Cathode., by Edward P. Thompson
*** END OF THIS PROJECT GUTENBERG EBOOK ROENTGEN RAYS AND PHENOMENA ***
***** This file should be named 63422-h.htm or 63422-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/6/3/4/2/63422/
Produced by deaurider, Barry Abrahamsen, and the Online
Distributed Proofreading Team at https://www.pgdp.net (This
file was produced from images generously made available
by The Internet Archive)
Updated editions will replace the previous one--the old editions will
be renamed.
Creating the works from print editions not protected by U.S. copyright
law means that no one owns a United States copyright in these works,
so the Foundation (and you!) can copy and distribute it in the United
States without permission and without paying copyright
royalties. Special rules, set forth in the General Terms of Use part
of this license, apply to copying and distributing Project
Gutenberg-tm electronic works to protect the PROJECT GUTENBERG-tm
concept and trademark. Project Gutenberg is a registered trademark,
and may not be used if you charge for the eBooks, unless you receive
specific permission. If you do not charge anything for copies of this
eBook, complying with the rules is very easy. You may use this eBook
for nearly any purpose such as creation of derivative works, reports,
performances and research. They may be modified and printed and given
away--you may do practically ANYTHING in the United States with eBooks
not protected by U.S. copyright law. Redistribution is subject to the
trademark license, especially commercial redistribution.
START: FULL LICENSE
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full
Project Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project
Gutenberg-tm electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or
destroy all copies of Project Gutenberg-tm electronic works in your
possession. If you paid a fee for obtaining a copy of or access to a
Project Gutenberg-tm electronic work and you do not agree to be bound
by the terms of this agreement, you may obtain a refund from the
person or entity to whom you paid the fee as set forth in paragraph
1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this
agreement and help preserve free future access to Project Gutenberg-tm
electronic works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the
Foundation" or PGLAF), owns a compilation copyright in the collection
of Project Gutenberg-tm electronic works. Nearly all the individual
works in the collection are in the public domain in the United
States. If an individual work is unprotected by copyright law in the
United States and you are located in the United States, we do not
claim a right to prevent you from copying, distributing, performing,
displaying or creating derivative works based on the work as long as
all references to Project Gutenberg are removed. Of course, we hope
that you will support the Project Gutenberg-tm mission of promoting
free access to electronic works by freely sharing Project Gutenberg-tm
works in compliance with the terms of this agreement for keeping the
Project Gutenberg-tm name associated with the work. You can easily
comply with the terms of this agreement by keeping this work in the
same format with its attached full Project Gutenberg-tm License when
you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are
in a constant state of change. If you are outside the United States,
check the laws of your country in addition to the terms of this
agreement before downloading, copying, displaying, performing,
distributing or creating derivative works based on this work or any
other Project Gutenberg-tm work. The Foundation makes no
representations concerning the copyright status of any work in any
country outside the United States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other
immediate access to, the full Project Gutenberg-tm License must appear
prominently whenever any copy of a Project Gutenberg-tm work (any work
on which the phrase "Project Gutenberg" appears, or with which the
phrase "Project Gutenberg" is associated) is accessed, displayed,
performed, viewed, copied or distributed:
This eBook is for the use of anyone anywhere in the United States and
most other parts of the world at no cost and with almost no
restrictions whatsoever. You may copy it, give it away or re-use it
under the terms of the Project Gutenberg License included with this
eBook or online at www.gutenberg.org. If you are not located in the
United States, you'll have to check the laws of the country where you
are located before using this ebook.
1.E.2. If an individual Project Gutenberg-tm electronic work is
derived from texts not protected by U.S. copyright law (does not
contain a notice indicating that it is posted with permission of the
copyright holder), the work can be copied and distributed to anyone in
the United States without paying any fees or charges. If you are
redistributing or providing access to a work with the phrase "Project
Gutenberg" associated with or appearing on the work, you must comply
either with the requirements of paragraphs 1.E.1 through 1.E.7 or
obtain permission for the use of the work and the Project Gutenberg-tm
trademark as set forth in paragraphs 1.E.8 or 1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any
additional terms imposed by the copyright holder. Additional terms
will be linked to the Project Gutenberg-tm License for all works
posted with the permission of the copyright holder found at the
beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including
any word processing or hypertext form. However, if you provide access
to or distribute copies of a Project Gutenberg-tm work in a format
other than "Plain Vanilla ASCII" or other format used in the official
version posted on the official Project Gutenberg-tm web site
(www.gutenberg.org), you must, at no additional cost, fee or expense
to the user, provide a copy, a means of exporting a copy, or a means
of obtaining a copy upon request, of the work in its original "Plain
Vanilla ASCII" or other form. Any alternate format must include the
full Project Gutenberg-tm License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works
provided that
* You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is owed
to the owner of the Project Gutenberg-tm trademark, but he has
agreed to donate royalties under this paragraph to the Project
Gutenberg Literary Archive Foundation. Royalty payments must be paid
within 60 days following each date on which you prepare (or are
legally required to prepare) your periodic tax returns. Royalty
payments should be clearly marked as such and sent to the Project
Gutenberg Literary Archive Foundation at the address specified in
Section 4, "Information about donations to the Project Gutenberg
Literary Archive Foundation."
* You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or destroy all
copies of the works possessed in a physical medium and discontinue
all use of and all access to other copies of Project Gutenberg-tm
works.
* You provide, in accordance with paragraph 1.F.3, a full refund of
any money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days of
receipt of the work.
* You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project
Gutenberg-tm electronic work or group of works on different terms than
are set forth in this agreement, you must obtain permission in writing
from both the Project Gutenberg Literary Archive Foundation and The
Project Gutenberg Trademark LLC, the owner of the Project Gutenberg-tm
trademark. Contact the Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
works not protected by U.S. copyright law in creating the Project
Gutenberg-tm collection. Despite these efforts, Project Gutenberg-tm
electronic works, and the medium on which they may be stored, may
contain "Defects," such as, but not limited to, incomplete, inaccurate
or corrupt data, transcription errors, a copyright or other
intellectual property infringement, a defective or damaged disk or
other medium, a computer virus, or computer codes that damage or
cannot be read by your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium
with your written explanation. The person or entity that provided you
with the defective work may elect to provide a replacement copy in
lieu of a refund. If you received the work electronically, the person
or entity providing it to you may choose to give you a second
opportunity to receive the work electronically in lieu of a refund. If
the second copy is also defective, you may demand a refund in writing
without further opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO
OTHER WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT
LIMITED TO WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of
damages. If any disclaimer or limitation set forth in this agreement
violates the law of the state applicable to this agreement, the
agreement shall be interpreted to make the maximum disclaimer or
limitation permitted by the applicable state law. The invalidity or
unenforceability of any provision of this agreement shall not void the
remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in
accordance with this agreement, and any volunteers associated with the
production, promotion and distribution of Project Gutenberg-tm
electronic works, harmless from all liability, costs and expenses,
including legal fees, that arise directly or indirectly from any of
the following which you do or cause to occur: (a) distribution of this
or any Project Gutenberg-tm work, (b) alteration, modification, or
additions or deletions to any Project Gutenberg-tm work, and (c) any
Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of
computers including obsolete, old, middle-aged and new computers. It
exists because of the efforts of hundreds of volunteers and donations
from people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future
generations. To learn more about the Project Gutenberg Literary
Archive Foundation and how your efforts and donations can help, see
Sections 3 and 4 and the Foundation information page at
www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg Literary
Archive Foundation are tax deductible to the full extent permitted by
U.S. federal laws and your state's laws.
The Foundation's principal office is in Fairbanks, Alaska, with the
mailing address: PO Box 750175, Fairbanks, AK 99775, but its
volunteers and employees are scattered throughout numerous
locations. Its business office is located at 809 North 1500 West, Salt
Lake City, UT 84116, (801) 596-1887. Email contact links and up to
date contact information can be found at the Foundation's web site and
official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To SEND
DONATIONS or determine the status of compliance for any particular
state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations. To
donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic works.
Professor Michael S. Hart was the originator of the Project
Gutenberg-tm concept of a library of electronic works that could be
freely shared with anyone. For forty years, he produced and
distributed Project Gutenberg-tm eBooks with only a loose network of
volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as not protected by copyright in
the U.S. unless a copyright notice is included. Thus, we do not
necessarily keep eBooks in compliance with any particular paper
edition.
Most people start at our Web site which has the main PG search
facility: www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.